Presentation
The importance of microbial resources for the present and the future is undeniable in view of their use in a wide variety of fields such as agronomy, food security, the environment, health, and biotechnology. Ensuring biosecurity and ensuring trade traceability are key elements of the future bio-economy as defined in 2007 in the OECD Best Practice Guidelines for Biological Resource Centers.
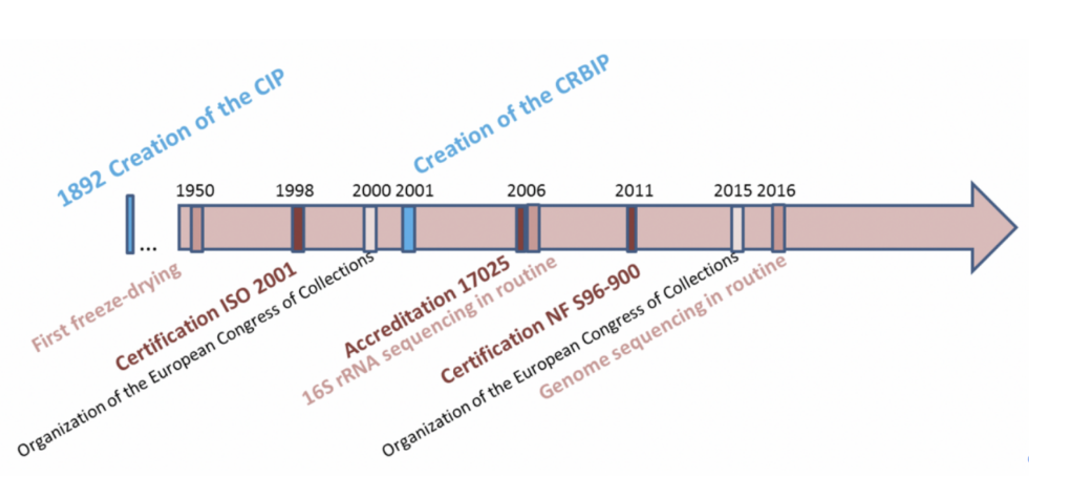
The Institut Pasteur Collection (CIP) was created by Dr. Binot, who started preserving bacterial strains as early as 1891. Freeze-drying began at the CIP in the 1950s. For more than a century now, locals of the CIP are on the campus of the Institut Pasteur.
The CIP has been able to constantly adapt to meet evolving user needs and current regulatory requirements. It is certified according to standard NF S 96-900.
CIP is rich of a beautiful biodiversity with more than 25,000 bacterial strains belonging to 5,500 different species. Since the 50's, the enrichment of the collection has been historically with:
- hospital bacterial strains (due to the proximity of Pasteur Hospital and Paris hospitals),
- bacteria from various National Reference Centers after the retirement of those responsible: Streptococci, Staphylococci, anaerobes, antibiotic resistant strains ...,
- typical strains of newly described bacterial species,
- bacteria from the environment.
The resources of the CIP are accessible via:
CIP-Catalogue : https://catalogue-crbip.pasteur.fr
The strains are preserved by lyophilization or freezing at -80 ° C or at -196 ° C in liquid nitrogen. As much as possible, two different methods of preservation and two types of storage for each strain are carried out in two different places.
Mission
The CIP's mission is to ensure the maintenance and enrichment of the Institut Pasteur's bacteria collection, firstly through collaborations with scientists from the Institut Pasteur and secondly through deposit of strains of French and foreign researchers. The CIP ensures the distribution of bacterial strains, the dissemination of information concerning distributed strains (properties, conservation, identification ...) and develops a research activity.
History
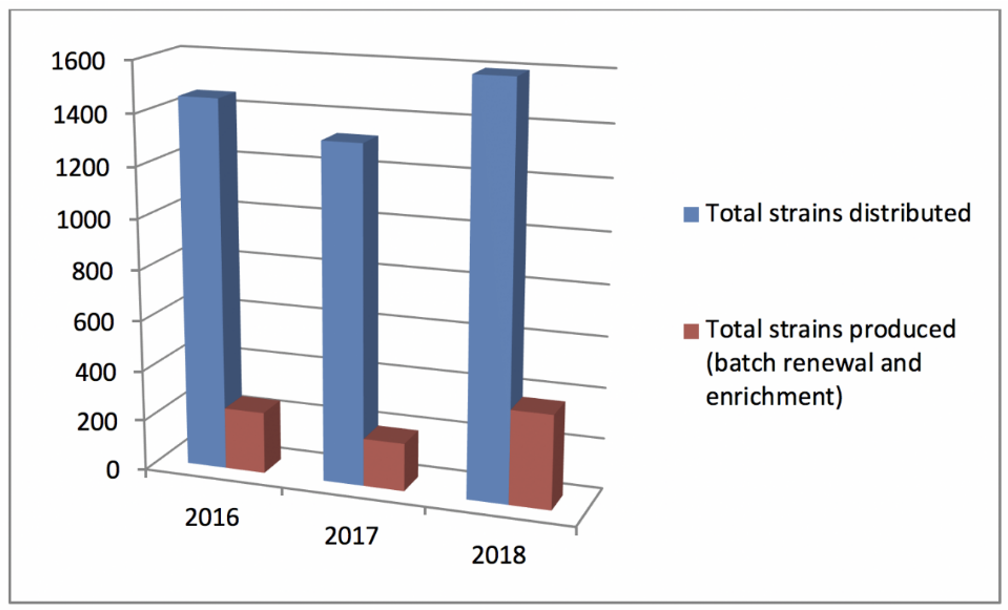
Statistics
Networks
The CIP is part of:
- Pasteur International Bioresources Network (PIBNet)
- BIOBANQUES
- European Culture Collections' Organisation (ECCO)
- Microbial Resources Research Infrastructure (MIRRI)
- World Federation for Culture Collections (WFCC)
- World Data Centre for Microorganisms (WDCM)
CIP RESEARCH
Collection de l’Institut Pasteur (CIP) | CIP Research
Research is varied because of the diversity of resources maintained in collection. Thus, the CIP is developing a research activity on identification, taxonomy (description of novel species), conservation, antibiotic resistance and metabolomics. For more information, please consult our research site.
CIP DEPOSIT
Collection de l’Institut Pasteur (CIP) | CIP DEPOSIT
General procedure for depositing a bacterial strain
The deposit of a bacterial strain is free of charge and is accompanied by a completed Deposit form for microorganisms with the information required for the implementation of the Nagoya Protocol (date of collection, country of origin, who collected the strain, …), the culture conditions, the components of the culture medium if necessary, the properties of the strain and the bibliography. The deposit form is to be sent to cip@pasteur.fr. The bacterial strain will only be sent after approval by the CIP, which reserves the right to refuse the deposit.
More information related to Nagoya Protocol on Access and Benefit-Sharing Clearing-House (ABSCH) website.
Deposit for publication of a new species
If you wish to obtain the genome sequencing of your strain, free of charge as part of collaboration, thank you kindly inform us when depositing it. If this collaboration is not desired, the deposit request should be formulated by attaching the publication to be submitted or the results of the phenotypic and molecular characterization of the strain. Thanks to this data, the CIP carries out the necessary checks in order to deliver the CIP number of the strain and its deposit certificate. The CIP undertakes not to disclose the information provided before the article is published.
FAQ
I sent my new strain to CIP along with the deposit form and the publication. How long will it take to get the deposit certificate I need for publication?
The CIP must carry out the necessary phenotypic and molecular controls for assignment of a CIP number and emission of the deposit certificate. As soon as the controls are performed, and the identification is confirmed, the deposit certificate is sent. This process usually takes between 4 and 8 weeks.
How can I send the bacterial strain?
You can send freeze-dried, frozen culture or as test tube culture in the appropriate medium and adequately labelled. We appreciate receiving in advance any specific cultivation condition that your strain might depend on. The material you send should be packed in a triple containment packaging system.
What happens if the country I isolated my strain is signatory of the Nagoya Protocol?
To meet the requirements of the Nagoya Protocol, it’s compulsory to provide with:
- The country and the exact location within the country where the genetic resource was collected (GPS coordinates) where the genetic resource was collected (not where it was isolated)
- The date of collection (sampling)
- The name of the person who collected
- The name of the national competent authority that granted access to the genetic resource as well as the documentation showing Prior Informed Consent (PIC) and Mutually Agreed Terms (MAT).
CIP DISTRIBUTION
Collection de l’Institut Pasteur (CIP) | CIP Distribution
The distribution of bacterial strains is limited to public or private institutions and educational institutions with a laboratory adapted to the handling of the strains ordered.
Order processing is done by the CIP Secretariat. The sending of biological materials is carried out in accordance with the regulations in force.
The strains are distributed for strictly scientific use (non-commercial) under a Material Transfer Agreement (MTA) which complies with our internal policy and the national and international standards.
To receive your quote or place an order
Browse the catalog of the CIP to find your need (or browse the reference strain catalog WDCM) and send your quote request or order by mail at : cip@pasteur.fr
Consult our price list here
Selection of strain of interest for public and private companies or Educational centers :
- Strains for antimicrobial resistance (link to come)
- Escherichia coli ECOR collection (link to come)
- Escherichia coli DEC collection (link to come)
- Escherichia coli recipient strains (link to come)
- Escherichia coli strains for colicin typing (link to come)
To place an order : Only for pasteurians
- Browse the CIP catalog
- Fulfill the "Internal tracability datasheet"
- Send it to cip@pasteur.fr
FAQ
I sent my new strain to CIP along with the deposit form and the publication. How long will it take to get the deposit certificate I need for publication?
The CIP must carry out the necessary phenotypic and molecular controls for assignment of a CIP number and emission of the deposit certificate. As soon as the controls are performed, and the identification is confirmed, the deposit certificate is sent. This process usually takes between 4 and 8 weeks.
How can I send the bacterial strain?
You can send freeze-dried, frozen culture or as test tube culture in the appropriate medium and adequately labelled. We appreciate receiving in advance any specific cultivation condition that your strain might depend on. The material you send should be packed in a triple containment packaging system.
What happens if the country I isolated my strain is signatory of the Nagoya Protocol?
To meet the requirements of the Nagoya Protocol, it’s compulsory to provide with:
- The country and the exact location within the country where the genetic resource was collected (GPS coordinates) where the genetic resource was collected (not where it was isolated)
- The date of collection (sampling)
- The name of the person who collected
- The name of the national competent authority that granted access to the genetic resource as well as the documentation showing Prior Informed Consent (PIC) and Mutually Agreed Terms (MAT).


