A fruitful international cooperation, scientists from the Institut Pasteur in Cambodia, the Institut Pasteur in Paris and the National Institutes of Health (NIH) developed the first in vitro test adapted to field conditions in malaria-endemic areas for the study of artemisinin-resistant Plasmodium falciparum (the parasite responsible for severe cases of malaria). Artemisinin is a major component in current antimalarial treatments. This test was developed for large-scale use to facilitate the surveillance of parasite resistance to antimalarial drugs. It is also the tool of choice for studying the biochemical and molecular basis of artemisinin resistance.
Press release
Paris, september 11, 2013
In order to prevent the spread of artemisinin-resistant parasites, effective tools must be used for their rapid identification. Until now, existing techniques did not make this possible. Clinical trials, which proved expensive and demanding for malaria patients, were the only option for detecting resistance. But now, it's a whole new ballgame, thanks to a simple test developed by scientists from the Malaria Molecular Epidemiology Unit at the Institut Pasteur in Cambodia in collaboration with teams from the Institut Pasteur in Paris and the NIH.
The test developed by the Institut Pasteur and NIH scientists makes it possible to study artemisinin-resistant forms of malaria, and because it is easy to implement it is perfectly adapted to field conditions in malaria-endemic areas and can be used on a large scale for surveillance purposes. This is how it works. First, a single blood sample is taken from a patient with malaria. Then, the parasites present in the blood are put into culture with a high dose of antimalarial treatment for a few hours. The degree of resistance is assessed three days later and is defined by the number of parasites that survive exposure to the antimalarial treatment. The higher the number, the more resistant the parasite is to the treatment.
Another version of this test to be used in research laboratories was also developed. This test opens doors to exciting new research opportunities that will allow scientists to take an in-depth look at the biological mechanisms used by resistant parasites and explore new active molecules to be used against artemisinin-resistant parasites. In this study, the scientists indicate that previous attempts to develop similar tests were unsuccessful because the concentrations of antimalarial drugs used were much lower than those to which parasites in treated patients are actually exposed.
The authors of this study were also able to use their test to determine, for the first time, at what stage of its development the Plasmodium falciparum parasite develops resistance to artemisinin derivatives. Their results showed that treatment was less effective on parasites in the very early stages of development.
Because of its ease of use and potential great contribution to the fight against malaria, the scientists from the Institut Pasteur in Cambodia, the Institut Pasteur in Paris and the NIH who collaborated to develop this test, wish to help implement its use throughout malaria-endemic areas. Initial plans for this test include a rapid distribution across the Asian continent with a primary objective of establishing a surveillance network and defining specific geographic zones where resistant parasites are present in order to adapt treatments and prevent spread to other malaria-endemic areas.
--
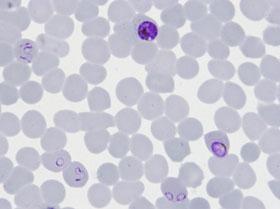
Illustration - Copyright Institut Pasteur
Caption - Blood smear, viewed under microscopy, showing the presence of Plasmodium falciparum parasites (purple) in human red blood cells.
Source
Novel phenotypic assays for the detection of artemisinin-resistant Plasmodium falciparum malaria in Cambodia: in-vitro and ex-vivo drug-response studies, The Lancet Infectious Diseases, September 11.
Benoit Witkowski (1), Chanaki Amaratunga (2), Nimol Khim (1), Sokunthea Sreng (3), Pheaktra Chim (1), Saorin Kim (1), Pharath Lim (1,2,3), Sivanna Mao (4), Chantha Sopha (5), Baramey Sam (6), Jennifer M. Anderson (2), Socheat Duong (3), Char Meng Chuor (3), Walter R. J. Taylor (7), Seila Suon (3), Odile Mercereau-Puijalon (8), Rick M. Fairhurst (2), Didier Menard (1).
(1) Malaria Molecular Epidemiology Unit, Institut Pasteur du Cambodge, Phnom Penh, Cambodia ;
(2) Laboratory of Malaria and Vector Research, National Institute of Allergy and Infectious Diseases,
National Institutes of Health, Bethesda, MD, USA ;
(3) National Center for Parasitology, Entomology and Malaria Control, Phnom Penh, Cambodia ;
(4) Sampov Meas Referral Hospital, Pursat, Cambodia ;
(5) Makara 16 Referral Hospital, Preah Vihear, Cambodia ;
(6) Ratanakiri Referral Hospital, Ratanakiri, Cambodia ;
(7) Service de Médecine Internationale et Humanitaire, Hôpitaux Universitaires de Genève, Geneva ;
(8) Parasite Molecular Immunology Unit, Institute Pasteur, Paris, France.
Contact
Service de presse de l’Institut Pasteur
Jérémy Lescène - Jeremy.lescene@pasteur.fr - +33 (0)1 45 68 81 01
Nadine Peyrolo - nadine.peyrolo@pasteur.fr - +33 (0)1 45 68 81 47