Antimicrobial resistance: curbing the health threat

Contents
Report - Antimicrobial resistance: curbing the health threat
- Growing resistance of pathogenic microbes
- The specter of a major global health crisis is now a reality
- The monkeypox virus and resistance to tecovirimat
- Aspergillus: resistance driven by agriculture
- Artemisinin effectiveness under threat from increasingly resistant malaria parasites
- How can we reverse the spread of antimicrobial resistance?
Understanding - The main antimicrobials and the diseases they target
Whooping cough - Unprecedented antibiotic resistance
Fungi - Candida auris: an invisible enemy in hospitals
Towards the end of the 19th century, Louis Pasteur showed that microbes are omnipresent – in water, in air, on objects, on the skin – and that some are responsible for disease. These pathogenic microbes can be divided into four broad families, each containing thousands of species: bacteria, viruses, parasites and fungi. Scientists and pharmaceutical companies have developed antimicrobial drugs and treatments to tackle the diseases they carry. The most familiar to us are antibiotics, used to tackle bacterial diseases. But antimicrobial drugs also include antivirals (against viruses), antiparasitics (against parasites or protozoa) and antifungals (against fungi).
Antimicrobials save millions of lives every year and have played a crucial part in improving human health.
Growing resistance of pathogenic microbes
Antimicrobial resistance (AMR) occurs when bacteria, viruses, fungi and parasites evolve over time and no longer respond to drugs, making it more difficult to treat infections and increasing the risk of disease spread.
Antibiotic resistance has become a major public health problem since antibiotics were introduced in the 1940s and began to be widely used. The rise in antibiotic resistance has been driven by overuse of antibiotics in human and veterinary medicine and in agriculture. This has caused bacteria to develop a wide range of survival strategies, including destroying or modifying antibiotics with enzymes, protecting the zones targeted by antibiotics, and expelling antibiotics with pump systems. Bacteria are able to transfer this arsenal of techniques among themselves, encouraging the spread of "superbugs." Although antibiotic resistance is inevitable, the current sharp rise is largely a result of human activity. And AMR is not limited to antibiotics – it has the potential to affect all treatments: antivirals (with resistant HIV strains), antifungals (with the emerging fungus Candida auris) and antimalarials (such as artemisinin, which is no longer effective against certain forms of malaria).
The main antimicrobials and the diseases they target
- Antibiotics for bacterial diseases: pneumonia, bronchitis, ear infections, meningitis, urinary tract infections, septicemia, chlamydia, gonorrhea, syphilis, Lyme disease, tuberculosis, leprosy, etc.
- Antivirals for viral diseases: HIV, influenza, herpes, chickenpox/shingles, cytomegalovirus, hepatitis B and C, RSV bronchiolitis, COVID-19, etc.
- Antimalarials against malaria, anthelmintics against parasitic worms and antiprotozoals against amebiasis, giardiasis, leishmaniasis, trypanosomiasis, toxoplasmosis, etc.
- Antifungals against fungal diseases: mycoses, infections that mainly affect the skin, hair, nails, mucous membranes and genital organs; several diseases affecting agricultural production, etc.
Unprecedented antibiotic resistance
In 2024, France experienced a major whooping cough outbreak with an estimated 150,000 cases, a level not seen for several decades. According to Sylvain Brisse, Head of the National Reference Center (CNR) for Whooping Cough at the Institut Pasteur, "this outbreak can be explained by two interconnected factors: the cyclical effect of the disease, and antigenic variations that facilitate immune evasion.
The outbreak also revealed an even more worrying phenomenon, with the emergence of strains resistant to macrolides, the main class of antibiotics used." Around 15 of the 400 strains analyzed at the CNR were resistant. "We discovered that the three French lineages had evolved from ancestors that were very close to Chinese strains."
This observation suggests multiple introduction events from China, where the current situation is particularly alarming: "Nearly all the whooping cough strains in China are resistant to macrolides, and this resistance is also spreading to other pathogens, no doubt as a result of excessive and inappropriate use of antibiotics." The CNR has developed a rapid molecular test (qPCR) to improve detection of the mutation responsible for resistance. "Using this test, we were able to estimate that the resistance rate in France is still low, just 2 to 3%."
Vaccination continues to be the most effective defense against whooping cough. There is a tendency among adults and elderly people to put off the recommended boosters, but this can lead to serious complications.
“Whooping cough: a contagious disease that should not be underestimated!” - Sylvain Brisse, Head of the French National Reference Center for Whooping Cough at the Institut Pasteur (Paris). Copyright: Jeanne Fenouil/ Institut Pasteur
The specter of a major global health crisis is now a reality
The consequences of antimicrobial resistance are serious and represent a major challenge for global health. Antimicrobial drugs are becoming less effective, and infections are now increasingly difficult to treat, or sometimes even entirely untreatable. Many medical procedures require antimicrobials:
- major surgery (especially implantable medical devices and transplants);
- cancer treatments such as chemotherapy;
- intensive care and neonatal care.
Without effective antimicrobial drugs, these procedures become much more dangerous.
The antimicrobial resistance crisis is already causing 4.95 million deaths each year (The Lancet, 2024), and this number could rise to 10 million by 2050. There is a risk that we will find ourselves in a post-antibiotic era, with common bacterial infections once again becoming fatal. This ominous prediction has raised alarm bells among leaders and authorities worldwide as resistance continues to spread at an alarming rate, driven by travel, trade and agriculture. A resistant microbe that emerges in one part of the world can soon spread across the planet. Resistance affects all countries, rich and poor alike, representing a major threat to global health security.
The monkeypox virus and resistance to tecovirimat
Mpox (formerly monkeypox) is an infectious disease caused by the monkeypox virus. It is mainly transmitted from rodents to humans, but it can also spread between humans. Since 2022, specific strains have caused major outbreaks that have spread outside traditionally endemic areas in Central and West Africa. The drug tecovirimat can be used to treat infected patients, but it is ineffective against certain resistant strains of the virus. In the United States in 2022, around 1% of patients treated with tecovirimat developed drug resistance. Research by scientists at the Institut Pasteur has shown how variants carrying certain mutations render antiviral treatment ineffective. Using data on the molecular structure of the viral protein targeted by tecovirimat, Institut Pasteur scientists led by Pablo Guardado-Calvo are developing new antivirals that could act on resistant viruses.

Candida auris: an invisible enemy in hospitals
Candida auris, retrospectively identified in South Korea in 1996 but first described as a novel species in 2009, is one of the most alarming emerging fungal pathogens. "The fact that it emerged almost simultaneously on several continents, with no epidemiological link, is striking," explains Alexandre Alanio, Deputy Director of the National Reference Center (CNR) for Invasive Mycoses and Antifungals at the Institut Pasteur. In France, it first appeared in 2007, identified retrospectively when the genomic database was updated by CNR Deputy Director Marie Desnos. It has now spread worldwide, with outbreaks reported from 2011 onwards, especially in India and the Middle East. "In Europe, cases are still imported, but we are seeing a worrying rise, especially in Southern and Eastern Europe, with transmission in healthcare settings."
Candida auris is able to colonize hospital environments, persisting on surfaces despite disinfection. In 2024, a French hospital experienced an outbreak of 25 patients infected with the same strain, indicating active transmission. Intensive care units are particularly vulnerable, as the fungus adheres to equipment and is not eliminated by standard cleaning practices. "Even with stricter cleaning protocols, it is hard to eliminate," says CNR Director Fanny Lanternier, emphasizing how healthcare settings act as an environmental reservoir conducive to reinfection, especially in immunocompromised patients.

Candida auris is resistant to several antifungal drugs, including azoles. "Candida auris strains in France are currently still sensitive to echinocandins, but resistance is emerging elsewhere, including in South America and the United States," warns Fanny Lanternier. If multidrug-resistant strains were to emerge, this would make treatment even more difficult.
The CNR plays a key role in surveillance and research. "We systematically sequence genomes to monitor strains and understand how they are spreading," explains Alexandre Alanio. These data, combined with those from hygiene specialists, are being used to adapt protocols in hospitals. "Our priority is to develop rapid diagnostic tools, especially for countries in the Global South, where resources are lacking," he continues. Fanny Lanternier adds: "Research on resistance mechanisms is crucial so that we can anticipate and prepare for crisis situations."
"Candida auris is a reminder that fungal pathogens don't stop at borders. It emerged as a result of global factors, and it needs a coordinated response," concludes Alexandre Alanio. Fanny Lanternier believes that "investing in research and surveillance is essential to stop the situation spiraling out of control." Urgent action is needed to curb the spread of this insidious pathogen, to avoid reaching a situation where there are no more effective treatments.
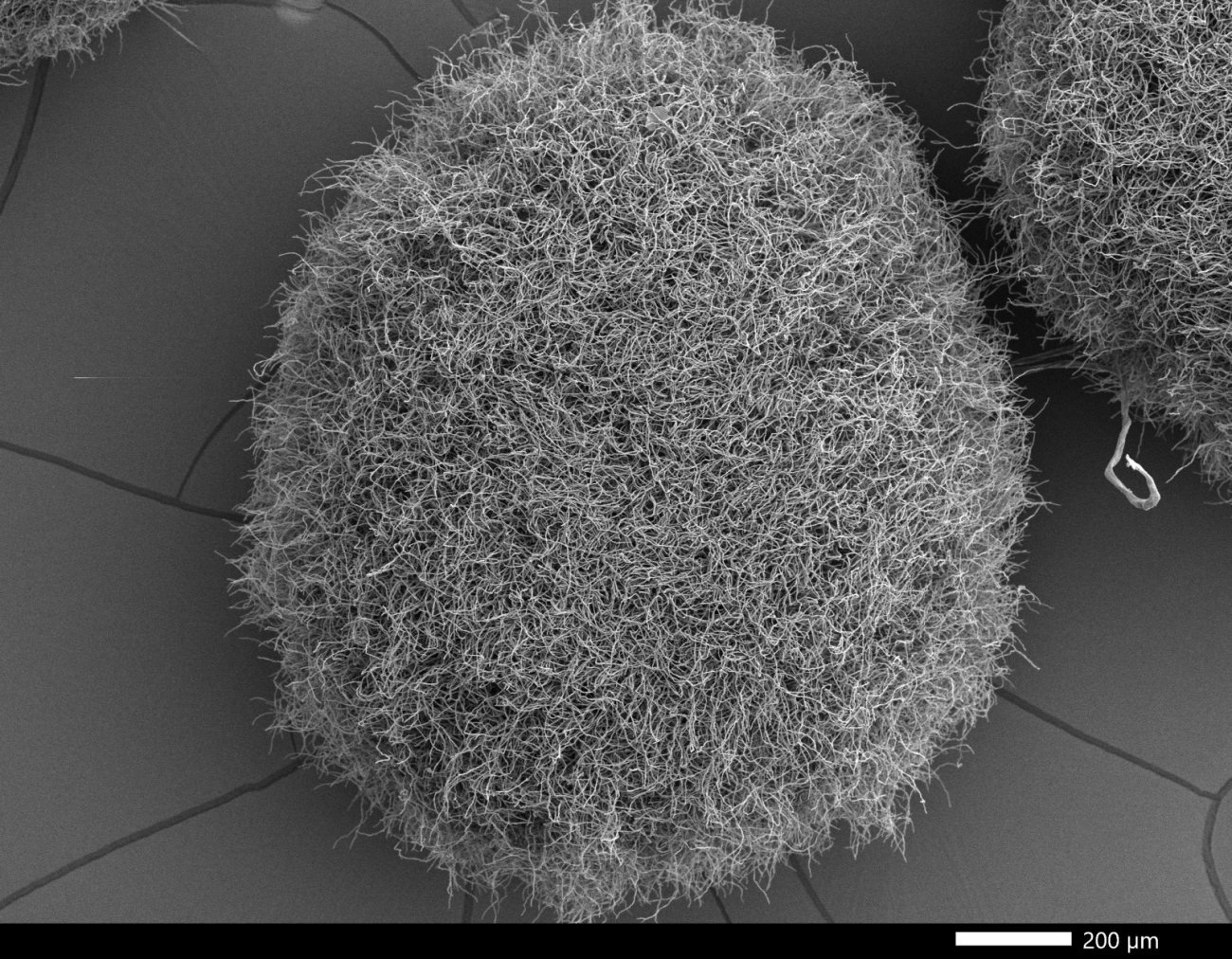
Aspergillus: resistance driven by agriculture
The fungus Aspergillus fumigatus, responsible for severe lung infections, is developing increasing resistance to first-line azole-based antifungal treatments. Azole-resistant Aspergillus infections, which represent up to 10% of cases in Europe, are associated with higher mortality in vulnerable patients, such as transplant recipients or those receiving chemotherapy.
Research by Alexandre Alanio (Institut Pasteur/AP-HP), one of the experts behind the recent EFSA (European Food Safety Authority) report, has revealed that resistance in Aspergillus is largely driven by environmental factors: widespread use of azole fungicides in agriculture promotes the selection of resistant mutant strains in soil, air or compost. These strains are then inhaled by humans, compromising the effectiveness of treatment in patients with weakened immune systems. The development of resistance in Aspergillus is monitored in France by the National Reference Center for Invasive Mycoses and Antifungals, especially Director Fanny Lanternier and Deputy Directors Dea Garcia-Hermoso and Alexandre Alanio.
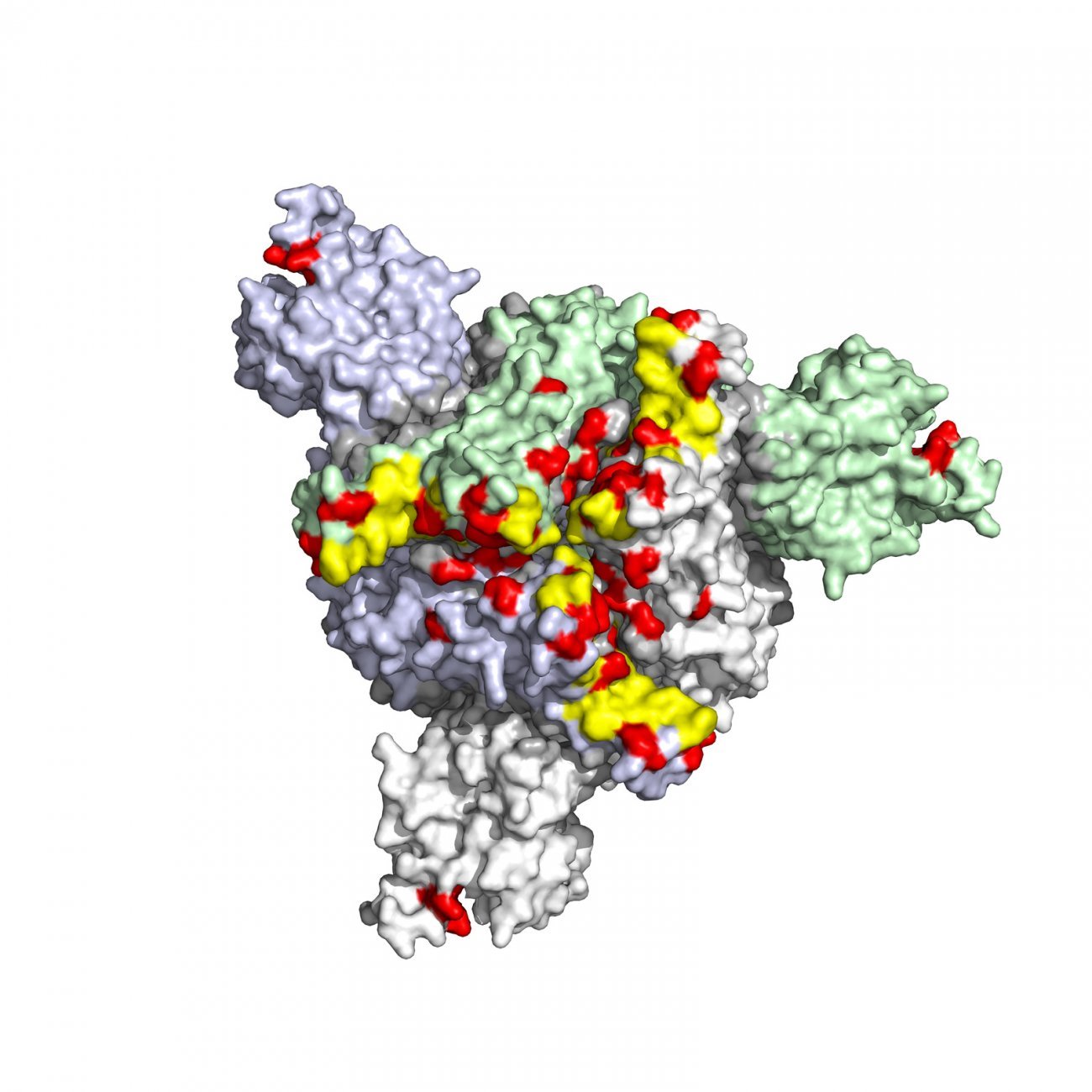
An underestimated threat?
Antiviral resistance receives less media coverage than antibiotic resistance, but it is a growing and underestimated challenge in the fight against viral infections. As Olivier Schwartz, Head of the Institut Pasteur's Virus and Immunity Unit, explains, "antiviral resistance is just as big a problem as antibiotic resistance, especially when it comes to rapidly evolving viruses like HIV and SARS-CoV-2." Viruses develop mutations in response to therapeutic pressure, enabling them to evade treatment. This is particularly worrying in the case of HIV, which requires lifelong treatment, and SARS-CoV-2, which rapidly developed variants that rendered the first antiviral monoclonal antibodies ineffective.
"A single mutation can be all it takes to induce resistance," continues Olivier Schwartz, emphasizing the importance of real-time monitoring.
To reduce this risk, many treatments are based on combinations of drugs, such as highly active antiretroviral therapy for HIV. Genomic sequencing can also be used to detect resistant mutations at an early stage and adapt treatment protocols as needed. Artificial intelligence offers new prospects for predicting resistance and designing more robust antivirals.
As Olivier Schwartz stresses, "without a detailed understanding of viral biology it will be impossible to develop sustainable treatments." A proactive approach is essential to preserve the effectiveness of these essential treatments. "We need to anticipate resistance by diversifying our therapeutic tools and stepping up surveillance of viral strains," summarizes Olivier Schwartz.
Artemisinin effectiveness under threat from increasingly resistant malaria parasites
A major challenge has emerged in the fight against malaria: the parasite Plasmodium falciparum, responsible for the most deadly form of the disease, is developing growing resistance to antimalarial drugs, especially artemisinin, the central component of the combination therapies recommended as the first-line treatment. Resistance was first observed in South-East Asia in the 2000s but is now also being seen in Africa, home to 90% of all reported malaria cases, with local emergence of resistant parasites.
Artemisinin resistance, elucidated following identification of the K13 genetic marker, represents a growing threat. K13 mutations delay elimination of P. falciparum from the bloodstream, reducing the effectiveness of treatments. In French Guiana, the only French territory with active indigenous transmission (a few hundred cases a year), the National Malaria Reference Center closely monitors these mutations to prevent their emergence and spread in forest regions. In the French overseas territories of Martinique, Guadeloupe and Mayotte, where malaria has been eliminated, resistance raises the risk of the disease being reintroduced if imported cases from Africa or Asia encounter competent mosquito vectors.

We need to devote more resources to fungal infections, because they can be serious and difficult to treat.
Fungal pathogens are often overlooked. What is their impact on human health?
Some fungi are responsible for serious infections in humans. Every year, between 1.6 and 2.6 million people worldwide die from fungal infections, and billions more are affected by skin or systemic infections. These figures are comparable to those for tuberculosis or some forms of cancer, but fungal pathogens are one of the poor relations of research, with less than 3% of the world's total infectious disease budget spent on them.
Fungal infections affect everyone. Fungal pathogens are everywhere: in the air we breathe, on our skin, even in our digestive system. They are an integral part of our environment, but they can represent a threat under certain conditions.
Which fungi are currently causing the most concern?
Four species in particular represent a real danger and are having an impact on public health. Cryptococcus neoformans is a fungus that my laboratory is studying closely. It is responsible for severe forms of meningitis, especially in people with weakened immune systems. If left untreated, these infections can be fatal.
Candida albicans is a natural part of our microbiota, particularly in the skin, digestive tract and mucous membranes. Under normal circumstances it is harmless, but if there is an imbalance in our microbial flora it can cause severe infections, such as candidemia (bloodstream infections) or deep-seated infections that are difficult to treat.
Aspergillus fumigatus is a fungus whose spores we breathe in every day, and they are harmless for most people. But in susceptible individuals they can cause pulmonary aspergillosis, a serious infection that destroys lung tissue and often resists conventional antifungal treatment.
Lastly, the fungus Candida auris is a source of growing concern for the scientific and medical community. It was only observed after 2005 and was not previously known to the scientific community. It is currently responsible for hospital-acquired infections in many countries.
How do fungi become resistant to antifungal drugs?
Antifungal resistance is a complex phenomenon that occurs as a result of two key mechanisms. The first is natural resistance – some fungi have genetic characteristics that offer them natural protection from the outset. The second mechanism is acquired resistance. This develops over time, particularly in patients receiving long-term treatment with antifungals. In response to this therapeutic pressure, the fungi mutate and become resistant. Another factor exacerbating the problem is the widespread use of antifungals in agriculture. The same classes of drugs are used to protect crops and treat humans. This intensive use encourages the emergence of resistant strains, which can then infect humans.
What treatments are available for fungal infections?
There are currently four main classes of antifungal drugs to treat fungal infections:
- Polyenes;
- Azoles;
- Echinocandins;
- 5-fluorocytosine.
New drugs are being developed, but it is likely that they will also be used in agriculture, which could accelerate the emergence of new resistance. The gaping hole in this therapeutic arsenal is a vaccine. Unlike bacteria and viruses, there are currently no vaccines to prevent fungal infections.
Is climate change influencing fungal infections?
Yes, and that is one of our main concerns. A warming climate could broaden the geographic distribution of certain fungi. Candida auris is a textbook case – its sudden emergence in several countries suggests that it may have adapted to higher temperatures, perhaps as a result of climate change. If this theory is confirmed, we could see the emergence of other fungal pathogens over the coming years.
Are we at risk of a fungal pandemic similar to COVID-19?
A fungal pandemic on the scale of COVID-19 is unlikely, as fungal infections do not spread as easily as viruses. The most worrying scenario would be the emergence of a fungus with high resistance, virulence and transmissibility. Although this is unlikely, scientists and physicians are keeping a close eye on the emergence of fungal pathogens, as such a situation could represent a major public health challenge.
Monitoring resistance markers
Resistance to artemisinin, a key malaria treatment, is mainly caused by mutations in the Kelch13 gene. These mutations reduce the parasite's ability to absorb hemoglobin, thus limiting the release of ferrous iron (Fe²⁺), which is needed to activate the drug. By blocking this mechanism, the parasite weakens the effect of artemisinin as if it were "defusing a bomb," according to Didier Ménard, a professor at the University of Strasbourg and scientist at the Institut Pasteur. But the resistance mechanism goes further than that: "Even within a genetically identical population, there are heterogeneous subpopulations. Some of these adopt a specific cellular program that gives them the ability to resist exposure to artemisinin."
Contrary to initial fears, the resistance observed in Africa is not linked to imported Asian strains; it is a local phenomenon that has emerged independently. "What's worrying is that resistance is emerging without any link between regions, which makes it much harder to control," explains Didier Ménard. Another challenge is that the hrp2 gene is being deleted in some parasites, preventing them from producing the HRP2 protein, the target of rapid diagnostic tests. This can lead to false negative tests for infected people, which can delay or prevent appropriate treatment. Various approaches are being adopted to tackle the growing difficulties in diagnosis and treatment: new drug combinations are being used, and surveillance is being stepped up to detect resistance as early as possible via molecular markers and in vitro testing.
How can we reverse the spread of antimicrobial resistance?
Combating antimicrobial resistance (AMR) requires a coordinated approach from scientists, physicians and veterinarians. Human health, animal health and environmental health are interconnected and must be protected together to prevent disease. This is what is known as the "One Health" concept. Given the global spread of resistance genes, for example via wastewater and international trade, if we fail to adopt a holistic approach we may see commonplace infections becoming deadly once again, as the World Health Organization (WHO) has warned. Cutting-edge multidisciplinary research and integrative approaches, involving epidemiology, genomics, evolutionary biology, modeling, and structural and chemical biology, are being used to understand how resistance emerges and spreads in microorganisms, and how it interacts with the host organism. The Institut Pasteur is actively monitoring emerging resistance through early warning systems in its National Reference Centers. It has also developed nanoelectronic sensors to detect antibiotic resistance in three minutes, and genomic tests to anticipate resistance to HIV or malaria. Scientists are also investigating treatment evasion strategies used by pathogens, which encourage resistance. They are particularly focusing on biofilms, a community of microbes that aggregate and attach to living or inert surfaces, producing a protective viscous matrix. The microbes in biofilms can be between a hundred and a thousand times more resistant to antimicrobials than microbes in a free state. Finally, scientists are developing alternative therapies such as phage therapy, anti-microbial peptides, novel drug combinations and bispecific antibodies.
The aim is to be constantly targeting the development of innovative therapeutic strategies (antibiotics, antiparasitics, antifungals, antivirals and anti-vector measures) to provide safer, more sustainable treatments for infectious diseases.