Human immunodeficiency virus (HIV) induces infected lymphocytes to synthesize an extracellular mesh that contains viral particles, and protects them against the immune system and antiretroviral drugs. This is the conclusion of an ANRS-supported study conducted Maria-Isabel Thoulouze at the Virology department of the Institut (Institut Pasteur/CNRS, UMR 3569 Paris) and her colleagues at Inserm and the Kremlin Bicêtre University Hospital. These results, which point to a new therapeutic target, will be presented in an oral communication on July 26, during the 9th Conference on HIV Science (IAS 2017), organized by the International AIDS Society and the ANRS in Paris from July 23rd to 26th 2017.
When isolated, HIV is a fragile virus of quite low infectivity, but it is easily transmitted from one cell to another when these cells are in contact. Very high doses of antiretrovirals are, therefore, needed to prevent infection. An ANRS-supported study conducted by Maria-Isabel Thoulouze at the Virology department (Institut Pasteur/CNRS) in collaboration with colleagues from Inserm and the Kremlin Bicêtre University Hospital, sheds new light on the mode of HIV transmission.
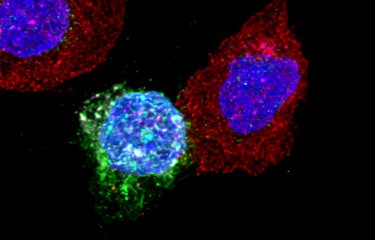
This study shows that HIV is transferred between lymphocytes mainly as highly infectious clusters of viral particles, transported in an adhesive extracellular matrix, which also shelters them from the immune system and antiretroviral drugs. “We have discovered that HIV is able to modify the secretion profile of infected lymphocytes, in order to form an external protective mesh containing viral particles,” says Dr Thoulouze. This protective mesh is composed principally of proteins and carbohydrates and is comparable to that of bacterial biofilm, an extracellular matrix network secreted by some bacteria as protection against their environment.
Using HIV-infected CD4+ T cells, Dr Thoulouze and colleagues analyzed the extracellular mesh formed at the surface of these cells and evaluated viral infection after its destruction. They then compared the infectivity of viral particles within the mesh and isolated viral particles, in the absence or presence of antiretrovirals, and assessed the efficacy of neutralizing antibodies against HIV. What they found is that “viral biofilm” formation increases HIV infectivity, reduces antiretroviral efficacy, and limits the action of antibodies. Compared with isolated viruses, those transported in this protective cocoon are also more readily transferred from one cell to another. “Thanks to this viral biofilm,” says Dr Thoulouze, “a cell is not infected by a single virus, but by colonies of several hundred viral particles, which may complement one another. Concentrated and compartmentalized in this way, HIV becomes less sensitive to drugs and less accessible to the immune system, which could explain how it persists in the body despite treatment.”
This extracellular mesh constitutes a new therapeutic target, destruction of which, Dr Thoulouze notes, could be a way “to limit the collective transport of viral particles and to increase the efficacy of the immune response to HIV and of antiretroviral therapies.” Further studies are needed to validate this new strategy.
Source
HIV-1 concentrates and shelters cell-associated infectivity a “viral biofilm” - IAS Conference (oral).
C. Inizan (1), A. Derames (1), M. Caillet (1), A. David (1), P. Versmisse (1), M. Mesel-Lemoine (1), P. Bomme (1), A. Mallet (1), H. Mouquet (1), F. Boufassa (2), O. Lambotte (3), K. Bourdic (3), A. Saez-Cirion (1), M.-I. Thoulouze (1)
1 Institut Pasteur/CNRS, Virology, Paris, France,
2 INSERM, CHU Kremlin Bicêtre, Kremlin Bicêtre, France,
3 CHU Kremlin Bicêtre, AP-HP, Le Kremlin Bicêtre, France.