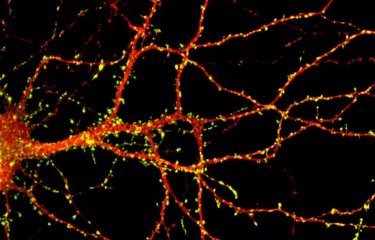
A team of researchers from I-STEM laboratory (CECS / AFM-Telethon / Inserm), led by Alexandra Benchoua and Marc Peschanski, in collaboration with Prof. Thomas Bourgeron (Pasteur Institute / Paris Diderot University / CNRS) and Prof. Richard Delorme (Robert Debré Hospital / AP-HP), has highlighted the therapeutic potential of lithium in a patient with a rare form of autism spectrum disorder associated with SHANK3 gene mutation. This molecule, usually used to treat bipolar disorder, was identified thanks to high throughput screening of chemical compounds on human neurons derived from pluripotent stem cells, including those of the treated patient. This study is a first step towards the development of tools for a more personalized medicine for individuals with autism spectrum disorders. This work was published May 27, 2016 in EBioMedicine review.
Autism spectrum disorders (ASD) represent a heterogeneous group of neurodevelopmental disorders that affect 1% of the population. They represent a challenge for the development of effective drug therapies because of the symptom complexity and variability between individuals. For about one third of patients with autism, a genetic cause has been identified. It was estimated than 1 to 2% of children with autism and intellectual disability are deficient for SHANK3 gene, a gene involved in Phelan-McDermid syndrome (the vast majority of patients with this syndrome have lost a fragment of chromosome 22 carrying a copy of SHANK3). It is to this population that was addressed the study. The loss of one of two copies of SHANK3 causes a decrease in the amount of SHANK3 protein and impairs the maintenance of the contact points between neurons (synapses). The aim of the researchers was therefore to identify drugs that can increase SHANK3 expression by activating the second still functional copy. However, SHANK3 being expressed only in neurons, it was first necessary to overcome a technological barrier to produce in vitro human neurons carrying the mutations of patients awaiting a treatment.
The researchers developed a strategy to produce at large scale human neurons from pluripotent stem cells. This allowed a high-throughput screening of pharmacological compounds to select those increasing the expression of SHANK3, therefore having the best chance of being efficient. Compounds identified were then tested on neurons of patients with SHANK3 mutations. Among the 202 tested compounds, lithium and valproic acid showed the best efficiency, restoring correct levels of SHANK3 and improving neuronal cells function. Lithium was then proposed to one of the patient after successful testing of the drug in vitro on its own neurons. After a year of treatment, this pilot study highlights an encouraging decrease of the severity of autism for this patient. Researchers are now considering conducting a double-blind randomized trial to confirm these results in a larger number of patients with autism spectrum disorders linked to mutations in SHANK3.
More broadly, this study shows that neurons derived from pluripotent stem cells offer a relevant cell model for the research of specific treatments for genetic forms of ASD. This methodology paves the way for precision medicine for psychiatric and neurological diseases.
This work was supported by, the National Research Agency, the Bettencourt-Schueller Foundation, the Conny-Maeva Foundation, the Cognacq Jay Foundation, Orange Foundation, Fondamental Foundation, Servier laboratories and by the AFM-Telethon through Telethon donations.
Source
Human pluripotent stem cell-derived cortical neurons for high throughput medication screening in autism: a proof of concept study in SHANK3 haploinsufficiency syndrome, Ebiomedicine, May 27, 2016.
Hélène Darville1, Aurélie Poulet1, Frédérique Rodet-Amsellem2, Laure Chatrousse1, Julie Pernelle3, 4, Claire Boissart1, Delphine Héron5,6, Caroline Nava5,6, Anselme Perrier3,4, Margot Jarrige3,4, Francis Cogé7, Mark J Millan7, Thomas Bourgeron8,9,10, Marc Peschanski3,4, Richard Delorme2, 8 and Alexandra Benchoua1
1 CECS, I-STEM, AFM, 91030 Evry Cedex, France;
2 Assistance Publique-Hôpitaux de Paris, Robert Debré Hospital, Department of Child and Adolescent Psychiatry, Paris, France
3 INSERM UMR 861 I-STEM AFM, 91030 Evry Cedex, France
4UEVE UMR 861 I-STEM AFM, 91030 Evry Cedex, France
5 Assistance Publique-Hôpitaux de Paris, Department of Genetic an Cytogenetic, Pitié-Salpétrière Hospital, 75013 Paris, France
6 Sorbonne Universités, UPMC Univ Paris 06 UMR S 1127, Inserm U 1127, CNRS UMR 7225, and ICM, F- 75013 Paris, France
7 IDR Servier, Croissy sur Seine, France
8 Institut Pasteur, Human Genetics and Cognitive Functions Unit, Paris, France
9 CNRS UMR 3571: Genes, synapses and cognition, Institut Pasteur, Paris, France
10 University Denis Diderot, Sorbonne Paris Cité, Paris, France