French Bioethics Law: an original participatory approach for the National Bioethics Consultation

A bioethics bill was tabled at the French Council of Ministers on Wednesday July 24, 2019. As the bill is examined by the French Parliament in September, it is a good opportunity to look at how this process came about and examine the contribution made by the French National Consultative Ethics Committee (CCNE). The CCNE Chairman, Professor Jean-François Delfraissy, came to the Institut Pasteur on June 25 to speak about the National Bioethics Consultation held in 2018 and discuss the topic "science and society" with scientists. The National Bioethics Consultation, held with a view to revising the Bioethics Law, was an excellent opportunity to take a closer look at issues related to research and society. During his visit to the Institut Pasteur, Jean-François Delfraissy spoke about the method adopted for the consultation and explained how the citizens' debate enabled the CCNE to submit an informed opinion.
"What sort of world do we want to create for the future?" That was the question asked by the French National Consultative Ethics Committee (CCNE) when the National Bioethics Consultation was launched in 2018. "The aim of the National Bioethics Consultation was to encourage discussions on a variety of different topics, some of which were directly linked to recent advances in science and technology," said Professor Jean-François Delfraissy, Chairman of the CCNE, in an interview that he granted us in April 2018. "These included artificial intelligence, genetics research, the relationship between health and the environment, and the question of health data."
The idea behind the National Bioethics Consultation was to identify avenues for the revision of the French Bioethics Law (which dates from 1994, with new versions in 2004 and 2011). "The statutory periodic revision of the Bioethics Law provides an opportunity to hold regular discussions on ethical issues related to progress in medicine and biology," reiterated the government following the Council of Ministers session on July 24. The revision takes place every seven years, and the bill tabled on July 24, 2019 is part of this process.
The Bioethics Law lays down regulations in areas such as organ donation, medically assisted reproduction (MAR), prenatal diagnosis and also biomedical research. The legislative framework prohibits research on human embryos, for example (apart from exceptional cases when special permission is granted).
During the National Bioethics Consultation, the CCNE raised nine important topics for reflection by citizens and experts.
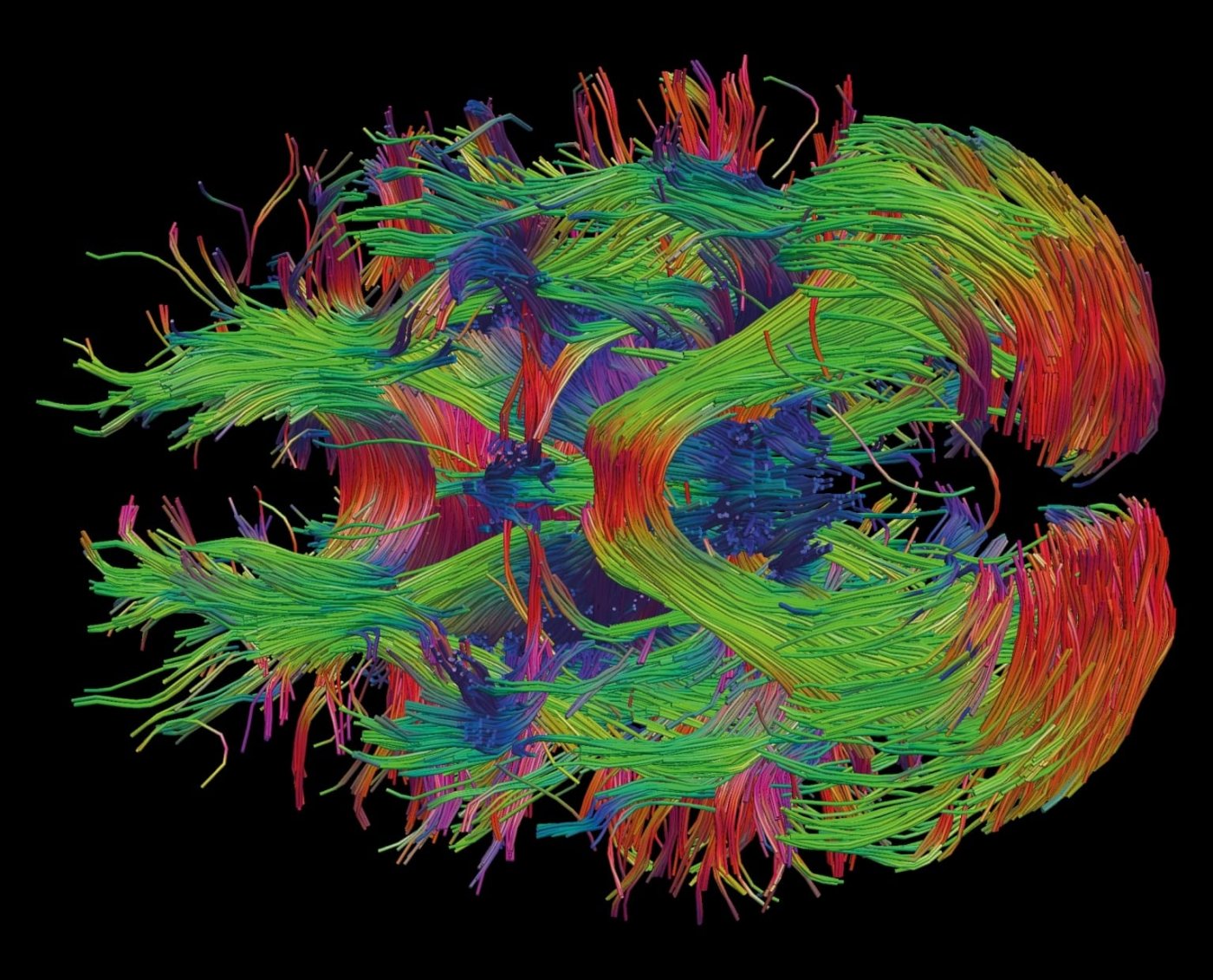
Neuroscience was one of the nine topics submitted for reflection by citizens and experts during the French National Bioethics Consultation in 2018. Here, an image of the reconstruction of brain connectivity in white matter, obtained using data from diffusion-weighted imaging. Copyright: Institut Pasteur / Roberto José Toro Olmedo.
The nine topics identified for reflection by citizens and experts
- Research on embryos and embryonic stem cells
- Genetic testing and genomic medicine
- Organ donations and transplants
- Neuroscience
- Health data
- Artificial intelligence and robotics
- Health and environment
- Reproduction and society
- End-of-life care
An innovative consultation method involving society
In 2018, the CCNE asked the public to share their thoughts on the topics identified for debate. "The consultation was designed to serve as a democratic public debate, an opportunity to consult both citizens and experts, encouraging them to express their views and engage in a dialog," explained Jean-François Delfraissy at the discussion session with Institut Pasteur scientists on the topic "science and society" on Tuesday June 25, 2019. To make sure this participatory exercise would produce effective results, the committee established various channels for consultation in mainland France and its overseas territories:
- a website (approximately 65,000 contributions and more than 800,000 votes);
- a citizens' committee (22 members took part in themed weekends);
- regional events (271 events were attended by 21,000 participants, a third of whom were students);
- hearings (with 154 organizations);
- meetings with institutional ethics committees.
The CCNE also guaranteed the transparency of the process by carefully moderating the website and setting up a citizens' committee to provide a critical perspective. "Our aim was for all the various means of communication to complement each other, and we tried to compensate the biases that are inherent in any form of consultation," continued Jean-François Delfraissy. The information provided to civil society was taken from the website and the debates held in all the regions and territories, for example, and an extensive series of hearings were held to enable experts, associations and organizations interested in bioethics issues (health insurance companies, think tanks, etc.) to air their views.
A largely positive conclusion, with some lessons to be learned
Following this broad national consultation, the CCNE expressed its satisfaction but also a number of reservations, acknowledging that any consultation method is imperfect and that it is only by considering all the communication channels together that an accurate picture of public opinion can be established. "Views on bioethics in France are strongly influenced by French culture and the country's solidarity-based healthcare system," explained Jean-François Delfraissy. "What emerged from the public consultation is that the many views expressed are not necessarily representative of public opinion. Various difficulties were encountered when it came to topics with societal relevance such as reproduction, and we did not always manage to include more vulnerable populations."
At the meeting of the French Council of Ministers on July 24, 2019 (page in French), a bill was tabled, laying down a series of legal regulations governing all medical and/or research practices. According to the French government website, the bill reflects a desire to "support free and responsible [biomedical] research that benefits human health, by removing various legal barriers and eliminating unjustified restrictions, especially when it comes to research on stem cells. At the same time, it reaffirms France's ethical values in the area of research, such as the ban on creating embryos for research purposes and changing the genetic heritage of embryos intended for pregnancy."
The bill also establishes a framework for a system of bioethics governance that will keep pace with advances in science and new techniques "by extending the missions of the National Consultative Ethics Committee (CCNE) for life sciences and health so that it considers the impact of any innovations on health."
An opinion delivered by the CCNE (Opinion 129) proposes guidance for political decision-makers on bioethics issues
Some of the nine topics discussed during the consultation have a direct impact on scientific research. The CCNE therefore sought the opinion of several research institutes, including the Institut Pasteur, a key player in biomedical research and innovation. Following the 2018 National Bioethics Consultation, during which it maintained a position of total neutrality, the CCNE was keen to express its own opinion (Opinion 129 - document in French) on both societal issues and matters related to progress in biomedical research.

Research on embryos and embryonic stem cells
There has been considerable progress in knowledge in the fields of reproduction, embryo development and the biology of embryonic stem cells since the late 1990s. Therapeutic developments in reproductive medicine, medically assisted reproduction and reparative medicine are therefore highly likely.
The CCNE therefore considers that the authorization of research on supernumerary embryos (preimplantation embryos from IVF procedures for which parental projects have been abandoned), including genetic modifications, is justified provided there is no embryo transfer. The CCNE also reiterates the ethical relevance of the ban on the creation of embryos for research purposes. The CCNE proposes using different legal regimes for embryo research and research on embryonic stem cell lines, as the ethical issues associated with these two types of research are different.
Genetic testing and genomic medicine
The development of new techniques of analysis and genomic engineering has recently led to new knowledge not only about the genome but also about the epigenome, with the use of new high-throughput sequencing techniques. The CCNE therefore suggests that preconception genetic diagnosis should be offered to everyone of childbearing age who so wishes, following genetic counseling. It also wishes to examine the possibilities of extending genetic testing to the general population by launching a pilot study to assess the consequences in terms of psychological impact and cost.
Neuroscience
The aim of neuroscience is to investigate both the functioning of the nervous system and also aspects such as behavior and mental processes. It is a field that relates to human identity itself, raising fundamental ethical questions. The CCNE therefore firmly opposes the use of functional MRI in the legal field. It also advises against use of functional MRI in "social" applications such as neuromarketing or for the selection of job applicants or insurance practices. Finally, it recommends that the general public should be given more information about these techniques.
Health data and artificial intelligence
The rapid growth of digital technologies within the healthcare system is a major issue and an irreversible trend. Digital science and technology play a vital role in the processing of information. The CCNE therefore recommends guaranteed human supervision of all use of digital technologies in health, and the obligation to put anyone who so wishes in contact at any time with someone able to provide them with information. It also considers that any person using artificial intelligence in the care pathway should be informed beforehand so that he or she can give free, informed consent. Finally, the CCNE proposes the creation in France of a secure national platform for the collection and processing of health data.
International
With the publication of Opinion 129, the CCNE sought the opinions of a number of foreign ethics committees at an international level (in Europe, North America and Japan). WHO and the Council of Europe are also advocates of holding citizens' debates on complex issues ("health democracy"). Japan is keen to set up a National Bioethics Consultation based on the French model to discuss various issues.
In conclusion, Professor Jean-François Delfraissy pointed out that reflection on bioethics issues does not end with the revision of the Bioethics Law, but that it should continue on an ongoing basis. The partnership with Regional Ethics Think Tanks will be a means of addressing some difficult topics in greater depth in the coming years..
Download the PDF of the CCNE report (in French)