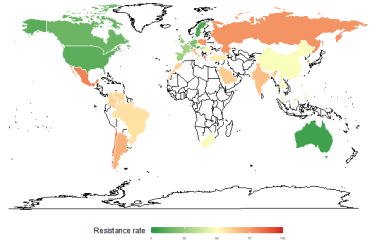
The Institut Pasteur's outstanding collection of bacterial strains has played a key role in research at the University of California. The collection was used in a study exploring how the modification of group A streptogramins, a class of antibiotics discovered and isolated in France in the 1960s, can restore their efficacy by enabling them to overcome the resistance mechanisms employed by the bacteria they are intended to destroy. The Institut Pasteur's supporting role in this study highlights the value of its unique collection of resistant strains and shows how useful it can be in this type of research. Antimicrobial resistance is one of the three priority scientific areas in the Institut Pasteur's 2019-2023 Strategic Plan.
In recent years virtually no new antibiotics have been developed; since the 1970s, the number of therapeutic classes available has remained around fifteen. "Antibiotics are derived from natural products made by microorganisms in the soil (such as fungi or filamentous bacteria like Streptomyces), which are used by humans because they are active against pathogenic bacteria and are not toxic for human or animal cells, so they are well tolerated," explains Olivier Chesneau, a scientist working for the Institut Pasteur Collection (CIP), created in 1891 to preserve bacterial strains. The CIP currently represents a wealth of biodiversity, with more than 25,000 bacterial strains belonging to 5,500 different species.
Research by Californian chemists to modify streptogramins
The pharmaceutical industry has developed antibiotic molecules through a process known as semisynthesis, whereby a compound is isolated by fermentation, then chemically modified to improve its pharmacokinetic properties (absorption and distribution in the body) and/or pharmacodynamic properties (effect on the pathogenic microorganism). Many of these modifications are designed to tackle resistance mechanisms developed over time by pathogenic bacteria – the aim is to modify the antibiotic so that it is not susceptible to resistance. But "it is becoming increasingly difficult to tackle the accumulation of resistance mechanisms in pathogenic bacteria," continues the Institut Pasteur scientist. "We are running out of chemical solutions to modify existing antibiotics and there is a real chance that we will find ourselves in a therapeutic impasse for several pathogens in the absence of new antibiotics."
In this context, the research by chemists at the University of California San Francisco (UCSF) is particularly innovative: they have designed a modular production process entirely based on the use of synthetic precursors, enabling tailored, large-scale modification of the group A component of streptogramins, a component that is vital for the biological efficacy of streptogramins but has a chemically complex structure (macrocyclic lactone with several double bonds).
Microbiological expertise from the Institut Pasteur
Streptogramins, isolated then brought to market in the early 1960s, are one of the last classes of antibiotics to have been discovered. They are mainly used in France, where they are the second-leading class of antibiotics consumed. They are based on the synergistic action of component A and component B, both of which bind to the bacterial ribosome to block it. The combination of the two components is bactericidal, making it highly effective. "At the Institut Pasteur we worked extensively on genes and mechanisms of resistance to streptogramins in the laboratories led by Névine El Solh and Patrice Courvalin." Olivier Chesneau previously worked in both units, and he now manages genetic resources for antibiotic resistance in the Institut Pasteur Collection. "I was contacted by the team of Californian chemists to provide microbiological expertise." The first task was to choose the resistant strains, then to test the efficacy of the candidate molecules in vitro before deciding on the most suitable animal model to assess the curative potential of the best candidate. "With this study we also helped promote the use of the molecule streptogramin in the United States and elsewhere, since France is virtually the only country with experience in this area. The biological material requested was strains of staphylococci and enterococci that were as diverse as possible and capable of producing all the known variants of an inactivating enzyme that acetylates and inactivates the A component of streptogramins."
A unique collection of resistant strains in the CIP
The US study, which initially drew on structural analyses identifying which areas of the A component interact with the ribosome and which interact with the acetylating enzyme, raised hopes that it would be possible to modify the area recognized by the acetylating enzyme without touching the active area blocking the activity of the bacterial ribosome. "The gamble paid off!" concludes Olivier Chesneau. "It was validated in vitro and in vivo!"
The Institut Pasteur's supporting role in this research boosted the visibility of its unique collection of resistant strains and demonstrated the considerable value of the collection for research in this field, especially since antimicrobial resistance is one of the three priority scientific areas in the Institut Pasteur's 2019-2023 Strategic Plan. "For streptogramins as well as other antibiotics," emphasizes Olivier Chesneau. "This is why we are involved in the CARE project led with Danish colleagues from DTU and French colleagues from the CIRM to develop a collection of resistant strains at European level."
Find out more about the CARE project
--------------------------------------------------------
Source
Synthetic group A streptogramin antibiotics that overcome Vat resistance, Nature, September 23, 2020
Authors: The study was led by Ian Seiple of UCSF. Qi Li, Jenna Pellegrino, D. John Lee, Arthur Tran, Hector A. Chaires, Ruoxi Wang, Jesslyn E. Park, Kaijie Ji, David Chow, Justin T. Biel, Matthew Jacobson, and James Fraser of UCSF; Axel Brilot and Kenneth Borrelli of UCSF and Howard Hughes Medical Institute; Na Zhang of UCSF and Beijing University of Technology; Gydo van Zundert of Schrödinger, Inc.; Dean Shinabarger, Cindy Wolfe, and Beverly Murray of Micromyx; and Estelle Mühle and Olivier Chesneau of Institut Pasteur are also co-authors.
Funding: This project was funded by the UCSF Program for Breakthrough Biomedical Research, funded in part by the Sandler Foundation (J.S.F. and I.B.S.), a Sanghvi-Agarwal Innovation Award (J.S.F.), Packard Fellowships from the David and Lucile Packard Foundation (J.S.F. and I.B.S.), NIH GM123159 (J.S.F.), and NIH GM128656 (I.B.S.).
This study is part of the priority scientific area Antimicrobial Resistance of the Institut Pasteur's strategic plan for 2019-2023.
For more information, please visit