Researchers at the Institut Pasteur of Dakar and the Institut Pasteur in Paris have reconstructed chains of transmission of Ebola virus in the capital city of Guinea, from February to August 2014. The data show the positive impact of control measures on the evolution of the epidemic but highlight the challenges of Ebola control in large urban centres. The study is published in The Lancet Infectious Diseases.
Press release
Paris, January 23, 2015
An epidemic of Ebola of unprecedented magnitude is ongoing in parts of West Africa since December 2013, affecting for the first time large urban centres like Conakry, the capital city of Guinea. To better understand and characterize the transmission of the virus and improve control strategies, researchers at the Institut Pasteur of Dakar led by Amadou Sall and Institut Pasteur in Paris from the team of Simon Cauchemez (Mathematical Modelling of Infectious Diseases Unit) reconstructed the chains of transmission of Ebola in Conakry, from February to August 2014.
Interviews of patients and their families and neighbours done by teams from the Institut Pasteur of Dakar and their local partners made it possible to measure Ebola transmission in different settings and at different times in the epidemic. In March, funeral transmission constituted 15% of all transmissions and hospital transmission constituted 35%. These proportions dropped respectively to 4% and 9% in April after safe burials were implemented and an Ebola treatment centre was opened. Patients that were admitted to the hospital infected on average twice less people in the community than those that were not. The study shows a majority of transmissions occurred in families or in the community and that the implementation of control measures had a substantial impact on the spread of Ebola in Conakry.
However, these intervention measures were insufficient to stop the epidemic as three epidemic peaks were observed between February and August: the first (February 24) was due to the introduction of a case from a city in the centre of Guinea (Dabola); the second (March 24) to cases that were not declared by a family; and the third (June 30) to the introduction of a case from Sierra Leone. Chains of transmission that started in Conakry also expanded to other parts of Guinea.
Finally, these investigations coupled with the analysis of biological samples indicated that the number of persons infected by a patient was positively correlated with the viraemia of the patient (quantity of viral particles in the blood).
By detailing the conditions of Ebola transmission, these analyses highlight challenges that need to be addressed to contain Ebola in large urban centres and may help improve the response in the field. In the future, these epidemiological data will be correlated with viral sequences collected in the field and that are currently being analysed by researchers at the Institut Pasteur and in the International Network of Pasteur.
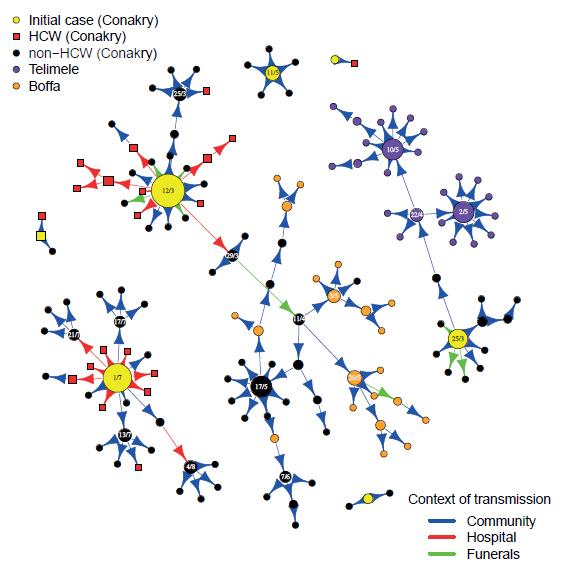
Figure: Transmission tree of Ebola virus. Dates of symptom onset are indicated in the circles for cases that infected more than 3 persons. The size of the circles is proportional to the number of persons infected by the case. © Institut Pasteur
Source
Chains of transmission and control of Ebola Virus Disease in Conakry, Guinea in 2014, The Lancet Infectious Diseases, January 23, 2015
Ousmane Faye (1,†), Pierre-Yves Boëlle (2,3,†), Emmanuel Heleze (4), Oumar Faye (1), Cheikh Loucoubar (1), N’Faly Magassouba (5), Barré Soropogui (5), Sakoba Keita (6), Tata Gakou (6), El Hadji Ibrahima Bah (7), Lamine Koivogui (8), Amadou Alpha Sall (1,‡), Simon Cauchemez (9,‡)
(1) Arbovirus and viral hemorrhagic fever Unit, Institut Pasteur de Dakar, Dakar, Senegal
(2) INSERM, UMR-S 1136, Institut Pierre Louis d’Epidemiologie et de Sante Publique, Paris, France
(3) Sorbonne Universités, UPMC Univ Paris 06, UMR-S 1136, Institut Pierre Louis d’Epidemiologie et de Santé Publique, Paris, France
(4) World Health Organization
(5) Projet de fièvres hémorragiques de Guinée, Université Gamal Abdel Nasser, Conakry, Guinée
(6) Ministry of Health, Conakry, Guinea
(7) Service des maladies infectieuses, Hopital Donka, Conakry, Guinée
(8) Institut National de Santé Publique de Guinée, Conakry, Guinée
(9) Mathematical Modelling of Infectious Diseases Unit, Institut Pasteur, Paris, France
† These authors contributed equally.
‡ Equal senior contribution.