Scientists at the Pasteur Institute in Paris and the CNRS (French National Center for Scientific Research), in collaboration with the Universities of Basel (Switzerland) and Cambridge (UK) have identified the mechanism underlying the formation of Buruli ulcers caused by the bacterium Mycobacterium ulcerans. Their discovery opens avenues for the development of novel therapeutic approaches for combating this disfiguring skin disease. This study is published online by The Journal of Clinical Investigation.
Press release
Paris, march 18, 2013
The destruction of cutaneous tissue is due to a lipid toxin called mycolactone that is secreted by the bacilli. Now, thanks to research led by Caroline Demangel at the Pasteur Institute in Paris in collaboration with Marie-France Carlier at the CNRS in Gif-sur-Yvette (France), the molecular target and mechanism of action of this toxin have been identified.
Caroline Demangel’s group together with collaborators at the Pasteur Institute and at the Universities of Basel and Cambridge have shown that mycolactone alters the architecture of epithelial cells by dysregulating the synthesis of their cytoskeleton, which is made of actin filaments. Cytoskeletal dynamics are essential in maintaining epidermal cell junctions, as well as the coordinated migration of cells towards sites of injury. By destabilizing the cytoskeleton, mycolactone therefore compromises both the integrity of cutaneous tissue and its potential to heal.
The current treatments for Buruli ulcer are based on intense antibiotic regimens, sometimes coupled with surgery to remove lesions, interventions that are poorly adapted to field conditions in regions where the disease is most prevalent. Blocking the ulcerative action of mycolactone could reduce current treatment regimes and eventually end them all together. The identification of the Wiskott-Aldrich syndrome proteins as molecular targets of mycolactone opens up original avenues of research towards the development of inhibitors that could provide alternative therapeutic options for the treatment of this disfiguring disease.
Financial support was provided by the Pasteur Institute, the French National Research Agency (ANR), the Raoul Follereau Foundation, the European Community and the French Cancer League. The Greater Paris region awarded a post-doctoral research fellowship to Romain Veyron-Churlet.
--
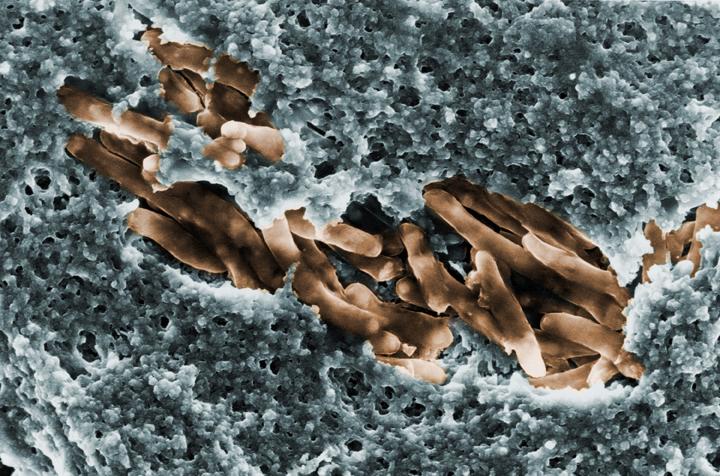
Illustration - © Laurent Marsollier / Institut Pasteur
Caption - Mycobacterium ulcerans, etiologic agent of Buruli ulcer, scanning electron microscopy.
Sources
Mycolactone activation of Wiskott-Aldrich syndrome proteins underpins Buruli ulcer formation, The Journal of Clinical Investigation, March 15, 2013.
Laure Guenin-Macé (1#), Romain Veyron-Churlet (1#), Maria-Isabel Thoulouze (2), Guillaume Romet-Lemonne (3), Hui Hong (4), Peter F. Leadlay (4), Anne Danckaert (5), Marie-Thérèse Ruf (6,7), Serge Mostowy (8), Chiara Zurzolo (9), Philippe Bousso (10), Fabrice Chrétien (11,12,13), Marie-France Carlier (3), and Caroline Demangel (1)
(1) Institut Pasteur, Immunobiology of Infection Unit, Paris, France
(2) Institut Pasteur, Lymphocyte Cell Biology Unit, Paris, France
(3) CNRS UPR 3089, Cytoskeleton Dynamics and Motility, Gif-sur-Yvette, France
(4) University of Cambridge, Department of Biochemistry, Cambridge, UK
(5) Institut Pasteur, Imagopole, Paris, France
(6) Molecular Immunology, Swiss Tropical and Public Health Institute, Basel, Switzerland
(7) University of Basel, Basel, Switzerland
(8) Institut Pasteur, Bacteria-Cell Interactions Unit, Paris, France
(9) Institut Pasteur, Membrane Traffic and Pathogenesis Unit, Paris, France
(10) Institut Pasteur, Dynamics of Immune Responses Unit, Paris, France
(11) Institut Pasteur, Human Histopathology and Animal Models Unit, Paris, France
(12) Faculty of Medicine, Université de Versailles Saint-Quentin-en-Yvelines, Versailles, France
(13) Department of Anatomical Pathology, Paris Public Hospital Network (AP-HP), Raymond Poincaré Hospital, Paris, France
# These authors contributed equally to this study.
Contact
Institut Pasteur Press Office
Nadine Peyrolo : + 33 (0)1 45 68 81 46
Jérémy Lescène : +33 (0)1 45 68 81 01
presse@pasteur.fr



