A team from the Institut Pasteur has discovered a new population of cells that develops around a tumor mass. This discovery opens up a new avenue of treatment for solid tumors. This offers hope for improving the treatment of cancers for which immunotherapies are often ineffective, such as pancreatic, breast and prostate cancer.
A malignant solid tumor is a localized mass that can invade surrounding tissues and even migrate to other organs. It is now well established that the course of a cancer is not determined solely by genetic mutations in tumor cells but by their interaction with the microenvironment. The tumor microenvironment is composed of healthy cells that the cancer has turned to its advantage. These include immune cells, blood vessels that supply the tumor and support cells known as fibroblastic stromal cells. In addition to ensuring its growth, this microenvironment protects the tumor from the body's antitumor immunity, in particular by preventing cytotoxic lymphocytes from infiltrating the tumor site and killing malignant cells.
Stromal cells protect tumors from the immune system
The team, led by Lucie Peduto, is focusing on fibroblastic stromal cells. These cells form a framework of connective tissue in all organs. By producing the extracellular matrix, such as collagen and regulatory factors, these cells ensure proper organ function and play an essential role in tissue repair.
As a tumor grows, stromal cells become activated, forming a protective womblike cocoon around the tumor. However, targeting these cells is risky, because while the cocoon protects the tumor from its host, it also protects the host from the tumor...
By studying the early stages of tumor growth, Lucie Peduto's team has identified a new stromal population that develops around the tumor, enabling it to evade the immune system. Unlike the ‘classical’ stroma, this stromal population, identified by the expression of a surface protein called ADAM12, is not involved in the formation of the protective cocoon. Instead, these cells regulate tumor macrophages and vascularization, thereby controlling the influx and activation of cytotoxic lymphocytes at the tumor site.
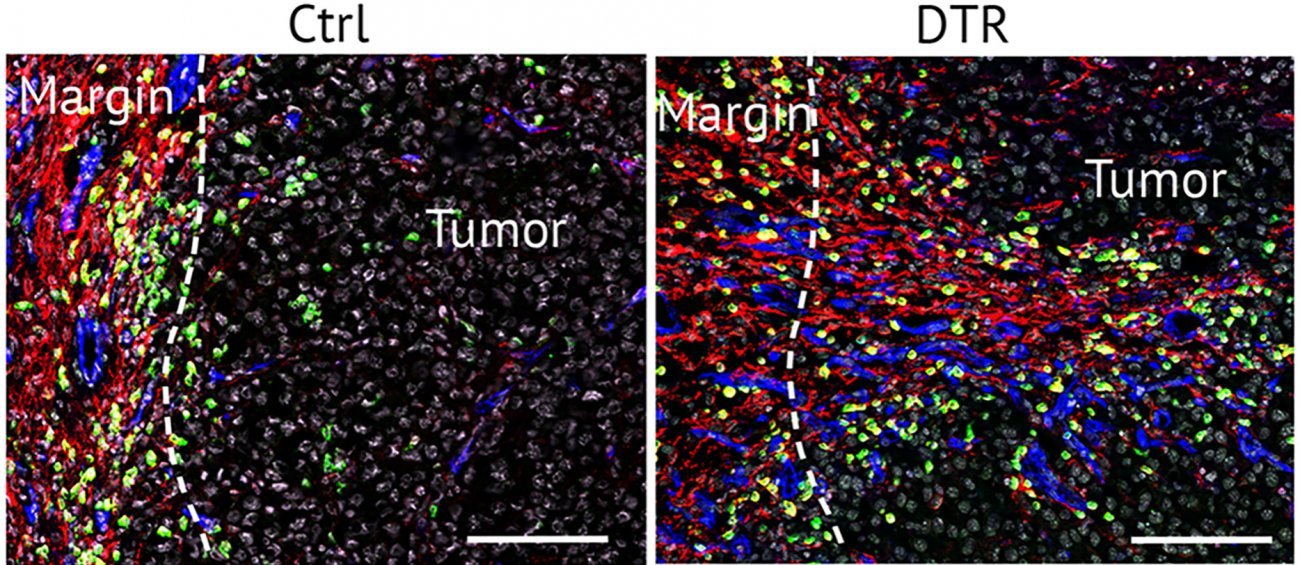
Tumor-induced ADAM12-expressing stromal cells are also present following injury. The tumor hijacks their repair function to protect itself. By promoting the action of tumor macrophages, these cells inhibit the immune system and promote formation of new blood vessels that develop anarchically. "Despite the abundance of blood vessels, tumor vessels are often not functional," explains Lucie Peduto. "This limits the influx of antitumor immune cells or therapeutic antibodies and creates a 'hypoxic' environment by reducing oxygen supply. Tumor hypoxia is a powerful resistance mechanism that inhibits the activity of antitumor immune cells such as T lymphocytes."
This makes it impossible for the body to defend itself against the tumor.
By eliminating stromal cells expressing ADAM12, Institut Pasteur researchers have demonstrated that it is possible to 'normalize' the tumor microenvironment, an essential step to improve access of activated cytotoxic lymphocytes within the tumor and block tumor growth.
A potential complementary treatment for solid tumors
The approach proposed by the Institut Pasteur team could improve treatments for solid tumors by specifically targeting stromal cells expressing the ADAM12 protein without destroying other beneficial stromal cells. Immunotherapies activate the immune system against the tumor. But this treatment currently is only poorly efficient for solid tumors such as pancreatic, breast or prostate cancer.
"The next step is to generate antibodies that specifically target these stromal cells and to combine them with immunotherapy to ensure more effective, targeted treatments," adds Lucie Peduto.
Current treatments such as chemotherapy destroy the tumor but also affect healthy parts of the body. The search for more targeted treatments is therefore a key aspect of medical research. This is the case with immunotherapies, which already target tumors but are unfortunately still ineffective against solid tumors. The discovery of a new target by the Institut Pasteur’s Stroma, Inflammation and Tissue Repair team is a game-changer.
This study is part of the Cancer Initiative of the Institut Pasteur's strategic plan for 2019-2023.
Source
Depletion of slow-cycling PDGFRα+ADAM12+ mesenchymal cells promotes antitumor immunity by restricting macrophage efferocytosis, Nature immunology, October 5, 2023
https://doi.org/10.1038/s41590-023-01642-7


