1% of all newborns – approximately 8,000 babies a year in France – are affected by a heart defect. 15% of these defects can be linked to a known genetic cause, but many remain unexplained. Defects affecting the alignment of cardiac chambers represent approximately 20% of heart defects in infants. A team of scientists from the Institut Pasteur and the UMR-1163 joint research unit at the Imagine Institute for Genetic Diseases (Inserm and Paris Descartes University) based at Necker Enfants Malades Hospital (AP-HP), led by a researcher from Inserm, recently elucidated a physical mechanism involved in the positioning of the four heart chambers during embryogenesis in mammals. The scientists designed a computer model which they used to generate a 3D reproduction of the morphogenesis process that turns the heart tube into a helix – the first stage in the arrangement of the chambers on the right and left side of the heart. These findings were published in eLife on November 28, 2017.
The heart is an organ that pumps blood throughout the body to supply oxygen and remove carbon dioxide and waste products. Its right and left sides are shaped differently to enable it to pump blood in a double circulatory system from the lungs to all the other organs and vice versa. In embryos, the heart initially resembles a relatively straight tube before forming a loop, similar to the characteristic helix shape of a snail shell, coiled anti-clockwise. This asymmetric form is important for the correct alignment of the cardiac chambers and the separation of the right and left sides of the heart, which in turn is vital for the double circulatory blood flow system. This stage of heart formation takes place during the first 20 to 28 days of human embryogenesis.
In an article published in the scientific journal eLife on November 28, 2017, the team led by Sigolène Meilhac, a scientist at Inserm and Head of the Heart Morphogenesis Laboratory (Institut Pasteur, Inserm, Paris Descartes University), revealed the mechanical constraints that govern the heart looping process. The scientists demonstrated that the developing heart undergoes two opposing forces at either end of the growing tube which are sufficient to turn the tube on itself and create the asymmetrical helix shape.
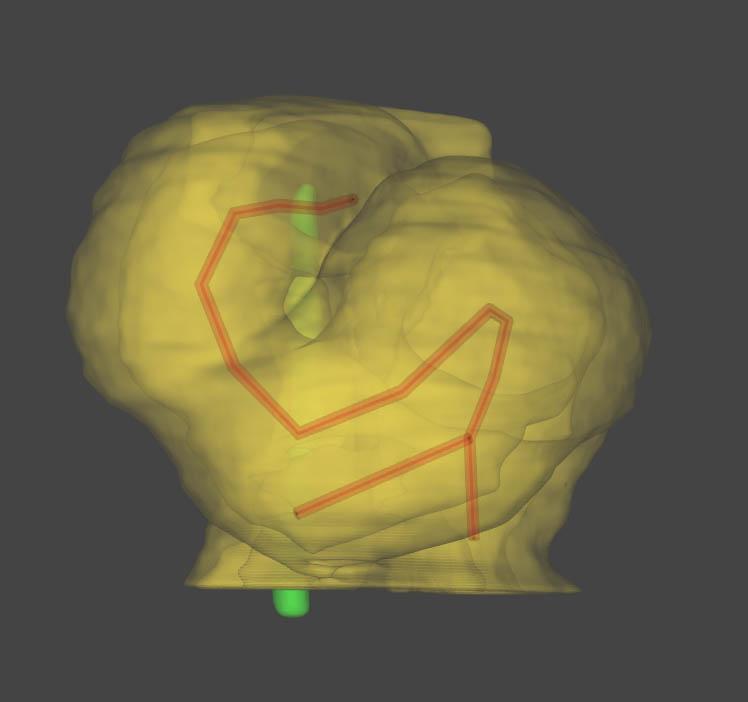
This multidisciplinary team, composed of a physicist, biologists and pediatric cardiologists, discovered that these constraints, which occur because of the way the heart is attached to the body, amplify the differences between cells from the right or left of the embryo. They used high-resolution episcopic microscopy and image quantification to provide 3D reconstructions of the shape changes and elucidate this stage of cardiac development for the first time in a mammal (a mouse). This innovative approach enabled them to design a computer model capable of predicting variations in heart formation depending on physical constraints or differences between right and left cells. The computer model was therefore able to accurately reproduce heart shape abnormalities observed in vivo.
These advances will better equip scientists to analyze abnormalities in cardiac morphogenesis. "By continuing our research into the genetic mechanisms of this stage of heart formation, we hope to identify new genetic mutations and shed light on the origins of heart defects in children. This research will be made possible by the synergy between the fundamental research carried out at the Institut Pasteur and the clinical and genetic research that is performed with AP-HP hospital patients on the Imagine Institute campus (Inserm/Paris Descartes University/AP-HP)," commented Sigolène Meilhac, lead author of the paper. The research will also provide novel insight into the mechanisms involved in the formation of other asymmetric organs such as the gut or the lungs.
Source
A predictive model of asymmetric morphogenesis from 3D reconstructions of mouse heart looping dynamics, eLife, November 28, 2017
Jean-François Le Garrec (1,2), Jorge N. Domínguez (3#), Audrey Desgrange (1,2#), Kenzo D. Ivanovitch (4,8), Etienne Raphaël (1,2), J. Andrew Bangham (5), Miguel Torres (4), Enrico Coen (6), Timothy J. Mohun (7) and Sigolène M. Meilhac (1,2 *)
(1) Imagine - Institut Pasteur, Laboratory of Heart Morphogenesis, 75015 Paris, France
(2) INSERM UMR1163, Université Paris Descartes, 75015 Paris, France
(3) Department of Experimental Biology, University of Jaén, CU Las Lagunillas, Jaén, Spain
(4) Cardiovascular Development Program, Centro Nacional de Investigaciones Cardiovasculares, CNIC, Spain
(5) University of East Anglia, Norwich, United Kingdom
(6) John Innes Centre, Norwich Research Park, Norwich, United Kingdom
(7) The Francis Crick Institute, London, United Kingdom
(8) Adresse actuelle : The Francis Crick Institute, London, United Kingdom
# These authors contributed equally to this study.