COVID-19 is a viral disease with respiratory symptoms that can induce fatal pneumonia. Understanding how SARS-CoV-2 spreads in the respiratory tract can help scientists identify the parameters controlling the severity of infection. Scientists from the Institut Pasteur, the CNRS and Inserm found that SARS-CoV-2 multiplies efficiently in the respiratory tract, and that it primarily targets ciliated cells in the epithelium and destroys their motile cilia. Virus-induced damage to cilia inhibits mucociliary clearance, a mechanism that expels inhaled particles and protects the respiratory tract from pathogens. The loss of cilia may help the virus spread to deeper regions of the respiratory tract, until it reaches pulmonary alveoli where it may induce pneumonia. This study is reported in an article published in Nature Communications on July 16, 2021.
The epithelium of the respiratory tract contains goblet cells, which secrete a viscoelastic mucus, and ciliated cells, which move the mucus layer by the coordinated beating of their motile cilia. Inhaled particles are trapped in the mucus layer and transported to the pharynx, where they are swallowed. The respiratory tract thus harbors a self-cleaning mechanism, known as mucociliary clearance, which prevents the accumulation of inhaled particles into the lungs.
The respiratory tract is the gateway for SARS-CoV-2, the virus responsible for COVID-19. In most people, infection with SARS-CoV-2 causes mild respiratory symptoms, but it can in severe cases lead to fatal respiratory distress. Understanding how SARS-CoV-2 spreads in the respiratory tract is crucial to help scientists identify the parameters underpinning the severity of COVID-19.
Scientists from the Institut Pasteur, the CNRS and Inserm examined the functional and structural consequences of SARS-CoV-2 infection in vitro using a bronchial epithelium model that mimics the natural lining of the airways. The model includes the three main cell types found in the airway epithelium: goblet cells, ciliated cells, and basal cells, which serve as local stem cells. The authors of the study observed that SARS-CoV-2 multiplies effectively in this system, and that it mainly targets ciliated cells. The multiplication of SARS-CoV-2 damages ciliated cells, which lose their motile cilia and are therefore no longer able to perform mucociliary clearance. These results were confirmed in an animal model, in collaboration with the teams of the NeuroCovid consortium at the Institut Pasteur.
"This research uncovers a process that may facilitate the spread of SARS-CoV-2 in the respiratory tract. The loss of cilia induced by viral infection may limit the clearance of locally produced viral particles, thereby allowing them to spread to deeper regions of the respiratory tract. Once viral particles reach the pulmonary alveoli, they can attack the pneumocytes and trigger pneumonia" explains Lisa Chakrabarti, joint last author of the study and Head of the Control of Chronic Viral Infections Group in the Virus and Immunity Unit led by Olivier Schwartz at Institut Pasteur.
The findings show that viral replication is also associated with early loss of the FoxJ1 transcription factor, which plays an important role in cilia formation and maintenance. The disruption of FoxJ1 regulation during SARS-CoV-2 infection may help explain the damage to the ciliary layer.
"Damage to cilia, which impairs mucociliary clearance, may also facilitate the spread of other inhaled respiratory pathogens, which may explain why patients who contract a severe form of COVID-19 often go on to develop secondary bacterial or fungal infections," explains Lisa Chakrabarti.
This study improves our understanding of pathogenic mechanisms induced by the original strain of SARS-CoV-2. The research will now be widened to include SARS-CoV-2 variants of interest, to explore the mechanisms underlying the increased infectiousness of these variants.
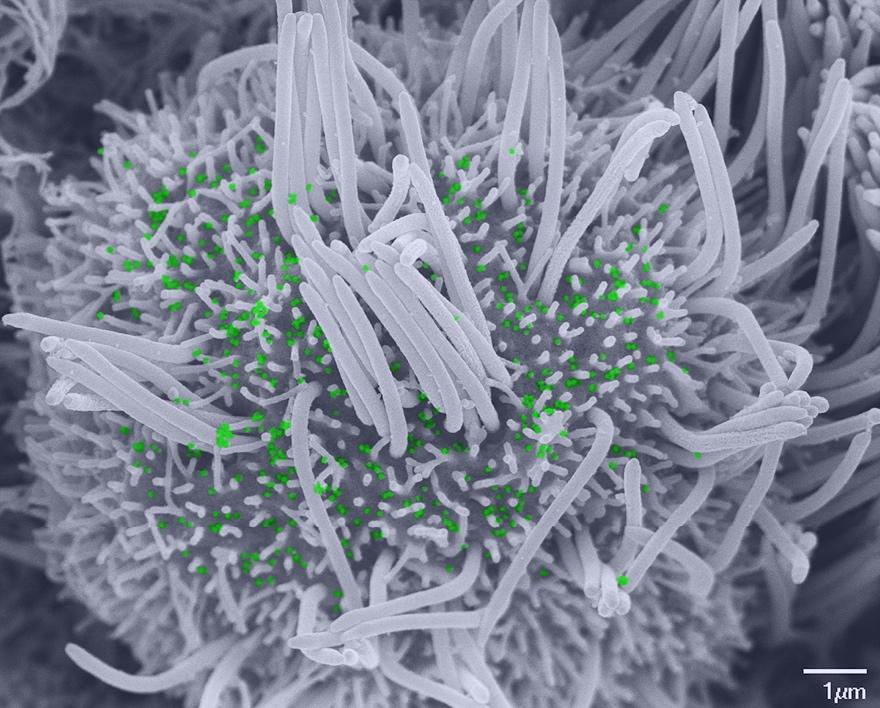
Scanning electron microscopy image showing a ciliated cell infected with SARS-CoV-2, with few remaining cilia and scattered viral particles (colored green) at the plasma membrane. © Vincent Michel, Rémy Robinot, Mathieu Hubert, Olivier Schwartz and Lisa Chakrabarti, Institut Pasteur
Source
SARS-CoV-2 infection induces the dedifferentiation of multiciliated cells and impairs mucociliary clearance, Nature Communications, July 16th, 2021
Rémy Robinot 1,2,16, Mathieu Hubert 1,2,16, Guilherme Dias de Melo 3, Françoise Lazarini 4,5, Timothée Bruel 1,2, Nikaïa Smith 6, Sylvain Levallois 7,8, Florence Larrous 3, Julien Fernandes 9, Stacy Gellenoncourt 1,2, Stéphane Rigaud 10, Olivier Gorgette 11, Catherine Thouvenot 11, Céline Trébeau 12, Adeline Mallet 11, Guillaume Duménil 11, Samy Gobaa 13, Raphaël Etournay 12, Pierre-Marie Lledo 4,5, Marc Lecuit 7,8,14, Hervé Bourhy 3, Darragh Duffy 6, Vincent Michel 12,*, Olivier Schwartz 1,2,15,* & Lisa A. Chakrabarti 1,2,*
1 Virus & Immunity Unit, Department of Virology, Institut Pasteur, Paris, France.
2 UMR 3569 CNRS, Paris, France.
3 Lyssavirus Epidemiology and Neuropathology Unit, Institut Pasteur, Paris, France.
4 Perception and Memory Unit, Institut Pasteur, Paris, France.
5 UMR 3571 CNRS, Paris, France.
6 Translational Immunology Lab, Department of Immunology, Institut Pasteur, Paris, France.
7 Biology of Infection Unit, Institut Pasteur, Paris, France.
8 INSERM U1117, Paris, France.
9 UtechS Photonics BioImaging, C2RT, Institut Pasteur, Paris, France.
10 Image Analysis Hub, C2RT, Institut Pasteur, Paris, France.
11 UtechS Ultrastructural BioImaging UBI, C2RT, Institut Pasteur, Paris, France.
12 Institut de l’Audition, Institut Pasteur, INSERM, Paris, France.
13 Biomaterials and Microfluidics Core Facility, Institut Pasteur, Paris, France.
14 Université de Paris, Necker-Enfants Malades University Hospital, Division of Infectious Diseases and Tropical Medicine, APHP, Institut Imagine, Paris, France.
15 Vaccine Research Institute, Créteil, France.
16 These authors contributed equally: Rémy Robinot, Mathieu Hubert.
* Corresponding authors


