Two teams from the Institut Pasteur in association with the French National Center for Scientific Research (CNRS) have published in the journal Nature the three-dimensional structure of two general anesthetics bound to their membrane receptor. This research provides the first atomic-resolution structures of general anesthetics which can be used to understand their action mechanism, a mechanism that has remained largely unknown since their discovery two hundred years ago. The research could therefore be a first step towards the development of new compounds that are more specific and cause fewer side effects.
Press release
Paris, january 20, 2011
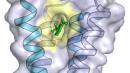
The scientists crystallized then used X-ray diffraction to analyze the complex which has never been observed before and which is formed by this channel and the anesthetics. To do this, they used a bacterial homolog of the GABAA channel, which they discovered in 2007*. The GABAA receptor is actually one of the main membrane targets for anesthetic molecules. In human beings, the GABAA receptor is largely responsible for transmission of inhibitory nerve impulses.
Using the atomic-scale resolution of the complex’s structure, the Institut Pasteur and CNRS scientists have been able to identify the specific binding sites to which the anesthetics bind. They have also been able to demonstrate that it is possible to adjust the affinity of the anesthetic for its receptor and the activity of the receptor by making a slight alteration to the configuration of the binding sites. These results support the data that are found in the scientific literature, whereby the binding of the anesthetic molecule increases the potential of the receptor’s inhibitory effect, thereby blocking even further the transmission of nerve impulses, such as the feeling of pain, for example.
This study is the first to show in high resolution the structural elements that underpin the action of general anesthetics. Although, with the use of ether, their discovery two hundred years ago revolutionized surgical practice, the mechanisms that explain how they work are not yet understood.
Reaching this key stage makes it possible to envisage using molecular modeling technologies to design and develop new molecules that are more specific and have reduced side effects.
- -
* See press release of November 5, 2008
Source
X-Ray structures of general anaesthetics bound to a pentameric ligand-gated ion channel, Nature, January 20, 2011.
Hugues Nury (1-4), Catherine Van Renterghem (1,2), Yun Weng (5), Alphonso Tran (5), Marc Baaden (6), Virginie Dufresne (1,2), Jean-Pierre Changeux (7,2-, James M. Sonner (5), Marc Delarue (3,4), Pierre-Jean Corringer (1,2)
(1) Institut Pasteur, Channel Receptors group, F-75015 Paris, France
(2) CNRS, URA 2182, F-75015 Paris, France
(3) Institut Pasteur, Structural Dynamics of Macromolecules unit, F-75015 Paris, France
(4) CNRS, URA 2185, F-75015 Paris, France
(5) Department of Anesthesia and Perioperative Care, University of California, San Francisco, USA
(6) Institut de Biologie Physico-Chimique, CNRS UPR 9080, 75005 Paris, France
(7) Institut Pasteur, F-75015 Paris, France
Contacts
Institut Pasteur Press Office
Marion Doucet - +33 (0)1 45 68 89 28 - marion.doucet@pasteur.fr
Nadine Peyrolo - +33 (0) 1 45 68 81 47 - nadine.peyrolo@pasteur.fr


