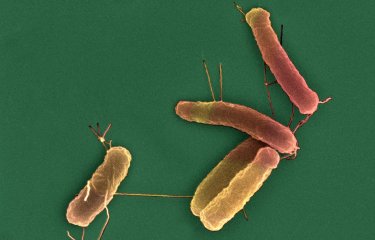
In a recently published study, scientists from the Institut Pasteur and its partners analyzed the genome of more than 700 strains of Escherichia coli bacteria to elucidate how antibiotic-resistant strains spread and evaluate the risk they represent.
Antibiotic resistance is a major public health challenge when it comes to tackling infectious diseases. The Institut Pasteur identified antimicrobial resistance as a priority scientific area in its 2019-2023 Strategic Plan. In a study published recently in the journal mSystems, scientists from the Institut Pasteur analyzed the resistance of Escherichia coli bacteria to carbapenems, a class of antibiotics, depending on their phylogeny and resistance to other antibiotics. This publication was produced in collaboration with the Paris Public Hospital Network (AP-HP) – Bicêtre Hospital, Université Paris-Saclay, CNRS, Inserm and Sorbonne Université.
Carbapenems are last-resort antibiotics that are mainly used to treat multidrug-resistant gram-negative bacilli, including E. coli and its family, the Enterobacteriaceae. The number of Enterobacteriaceae strains that are resistant to carbapanems has increased significantly in recent years, and the development of new antibiotics to tackle these pathogens is seen as an urgent priority by WHO. E. coli is both a research model and also one of the bacteria most frequently responsible for infections.
Three main categories of resistant strains
Carbapenem resistance is caused by the production of enzymes known as carbapenemases, which break down carbapenems. It is a major source of concern, given the ability of E. coli strains to spread in hospitals, healthcare facilities and also potentially among the general population. During the study, the scientists worked in collaboration with the Institut Pasteur's Biomics platform to sequence the genome of more than 700 carbapenemase-producing E. coli strains collected by the laboratory in the National Reference Center for Antibiotic Resistance, based at Bicêtre Hospital. A detailed bioinformatic analysis of these genomes was then used to classify the resistant strains of E. coli into three categories and to develop hypotheses as to their impact on public health:
- Multidrug-resistant strains with several other resistance genes as well as the carbapenemase gene and mutations that also contribute to their resistance to various antibiotics. These strains are capable of spreading and should be considered as at-risk clones that need to be monitored as a matter of priority.
- A dominant lineage of resistant strains that have also spread, in which the carbapenemase gene is integrated into the chromosome in a stable way, unlike other strains that carry it in a plasmid, an additional portion of DNA that is not necessary for bacterial survival. This contributes to the spread of these bacteria and reduces the fitness cost that the plasmid represents for E. coli. These strains are also considered as having high pathogenic potential.
- Strains that are sensitive to multiple drugs and seem to have recently acquired the plasmid carrying the carbapenemase gene from another bacterium. These strains do not represent a risk in therapeutic terms because they are sensitive to several antibiotics, but they could contribute to the spread of carbapenemase genes.
Among the strains analyzed, E. coli strains from at-risk lineages with high pathogenic potential that are often responsible for infections ultimately proved to be rare – an observation that was reassuring. In general, this research can be used to evaluate the risk associated with carbapenemase-producing E. coli strains and may help to optimize a surveillance strategy.
This study is part of the priority scientific area Antimicrobial Resistance of the Institut Pasteur's strategic plan for 2019-2023.
Source:
Specificities and Commonalities of Carbapenemase-Producing Escherichia coli Isolated in France from 2012 to 2015, mSystems, January 11, 2022
Rafael Patiño-Navarretea,b, Isabelle Rosinski-Chupina,b, Nicolas Cabanela,b, Pengdbamba Dieudonné Zongoa,b,c, Mélanie Hérya,d, Saoussen Oueslatia,d, Delphine Girlicha,d, Laurent Dorteta,d,e,f, Rémy A. Bonnina,d,f, Thierry Naasa,d,e,f, Philippe Glasera,b
a - Unité EERA, Institut Pasteur, APHP, Université Paris Saclay, Paris, France
b - UMR3525, CNRS, Université de Paris, Paris, France
c - Sorbonne Université, Paris, France
d - Team “Resist” INSERM U1184 “Immunology of Viral, Auto-Immune, Hematological and Bacterial diseases (IMVA-HB)”
e - Department of Bacteriology-Hygiene, Bicêtre Hospital, APHP, Le Kremlin-Bicêtre, France
f - Associated French National Reference Center for Antibiotic Resistance, Le Kremlin-Bicêtre, France