As we are already feeling the major impacts of climate change, we urgently need to gain a better understanding of the consequences of environmental transitions for human health. With its unique expertise in infectious and vector-borne diseases, the Institut Pasteur is working actively in this field.
Read on to find out more about some of the ways in which the environment is changing, biomedical research related to these shifts, and the Institut Pasteur's efforts to reduce its own environmental footprint.
Ticks, mosquitoes, and other vectors... How environmental transitions are impacting health
Mpox: the story of a (newly) emerging disease
- Deforestation and biodiversity loss
- The end of historical vaccine protection
- Travel and high-risk behavior
- A new strain of the virus
- Why is it wrong to use the term "monkeypox"?
- Afripox: an effective cooperative project
The rise of temperature: How climate change affects our bodies
Fungi threat: Pesticides encourage the emergence of resistance in fungi
- Which people are likely to contract a severe fungal infection? And why are we seeing more infections now than we used to?
- Is this resistance linked to misuse of antifungal drugs, like antibiotic resistance in bacteria?
- So why are we seeing resistance?
- So does climate change also play a part?
- Are there promising avenues for treatment?
Leishmaniasis, is not just a dog disease
The current environmental crises are exacerbating infectious diseases, especially vector-borne diseases
With: Sarah Bonnet, an INRAE scientist in the Ecology and Emergence of Arthropod-borne Pathogens Unit, and Philippe Bastin, Head of the Trypanosome Cell Biology Unit and scientific coordinator of the Research Center on Climate- and Environment-related Infections
Tick-borne diseases are a good example. "They are a perfect illustration of the One Health concept, which emphasizes the interdependent nature of human, animal and ecosystem health," explains Sarah Bonnet, an INRAE Research Director working in the Ecology and Emergence of Arthropod-borne Pathogens Unit at the Institut Pasteur. Ticks feed on the blood of their vertebrate hosts, which may include various animals and also humans. It is during these meals that they acquire pathogens (viruses, bacteria or parasites), which they can then pass on at their next blood meal. They potentially therefore serve as vectors for the agents responsible for diseases such as Lyme borreliosis, tick-borne encephalitis or rickettsial infections. The existence of this risk can raise complex questions, for example when it comes to greening urban areas. "The trend of introducing green spaces in towns and cities is necessary to adapt to climate change, but it can encourage ticks in urban environments," explains Sarah Bonnet. "So we are trying to determine the level of risk and identify solutions to minimize it."
Valérie Choumet, scientist at the Institut Pasteur, explains in 1 minute the recent breakthrough and discoveries on parle en 1 minute des avancées et récentes découvertes sur la Maladie de Lyme © Institut Pasteur
Scientists are also keeping a close eye on exotic ticks. "Some species, such as Hyalomma marginatum, which transmits the virus responsible for Crimean-Congo hemorrhagic fever (CCHF), travel with migratory birds," says Sarah Bonnet. "Until recently, conditions were not suitable for them to settle in Europe, although they were regularly imported. But this is changing as we are seeing longer drier summers and milder winters. H. marginatum populations have now acclimatized to the south of France, where the CCHF virus, previously thought to occur only in tropical countries, was detected in 2023."
New impetus for research
In 2028, a highly anticipated Research Center on Climate- and Environment-related Infections will open at the Institut Pasteur so that scientists can study this type of pathogen in more depth. Its four high-security laboratories (BSL3) will provide a unique interdisciplinary environment for studying pathogens and their vectors. "Fifteen teams will be working alongside each other in what will be the only facility of its kind in Europe," enthuses the project's scientific coordinator, Philippe Bastin. "The aim is to be even more responsive to the risks of new disease outbreaks."
The story of a (newly) emerging disease
With Antoine Gessain, Head of the Oncogenic Virus Epidemiology and Pathophysiology Unit (Institut Pasteur).
Mpox, previously known as monkeypox, hit the headlines in 2022 when it began to be exported outside the African continent. Given the speed with which it spread and the concerns it immediately raised, one could have been forgiven for thinking that it was an entirely new disease. But the first case was actually identified in 1970. Like many zoonoses – diseases transmitted by animals –, mpox may never have infected humans if various environmental changes had not led to closer contact between humans and wild animals.
Deforestation and biodiversity loss
Central Africa is home to the world's second largest rainforest, and like all rainforests it is under threat. First, climate change is reconfiguring its ecosystems, causing animal species to move. Second, exploitative logging and shifting cultivation are constantly gnawing at the edges of the forest, reducing its surface area. The result is that humans are suddenly finding themselves in close proximity to wild animals that until recently lived a much greater distance away. We are also witnessing a reduction in numbers of large game, which are now hunted not just to feed local villages but also to sell at markets in larger urban areas. Local populations have therefore had to turn to small mammals such as rodents for animal protein, and several squirrel species are suspected of being reservoirs of MPXV, the virus responsible for mpox. People who hunt them or pick fruit that they may have contaminated can become infected, and then spread the disease to family members living under the same roof.
Why is it wrong to use the term "monkeypox"?
MPXV was discovered in 1958 in laboratory monkeys – hence the fact that it was originally referred to as monkeypox. But although primates can be infected by the virus, they are not its natural reservoir. The natural reservoir for the virus is thought to be the Thomas's rope squirrel (Funisciurus anerythrus). Scientists from the Institut Pasteur in Paris and the French Museum of Natural History have revealed a strong correlation between the squirrel's range and places of infection.
"Transmission generally occurs through direct, close contact with skin lesions or with contaminated items, especially bedding," explains Antoine Gessain, a physician and virologist at the Institut Pasteur. That is why for several decades, despite regular outbreaks in villages on the outskirts of forests, mpox never became a pandemic.
In two of the most endemic countries, the Democratic Republic of the Congo (RDC) and the Central African Republic (CAR), another factor has contributed to the spread of infection: the impact of armed conflict, whether for short or long periods. Fighting causes populations to flee their villages and take refuge in forests, not only bringing them closer to animal reservoirs but also making it more difficult to treat patients.
The end of historical vaccine protection
Mpox is clinically similar to smallpox. The symptoms are also relatively comparable: flu-like signs such as fever, chills, aches, headache and tiredness, skin lesions such as vesicles or pustules, and swollen lymph nodes.
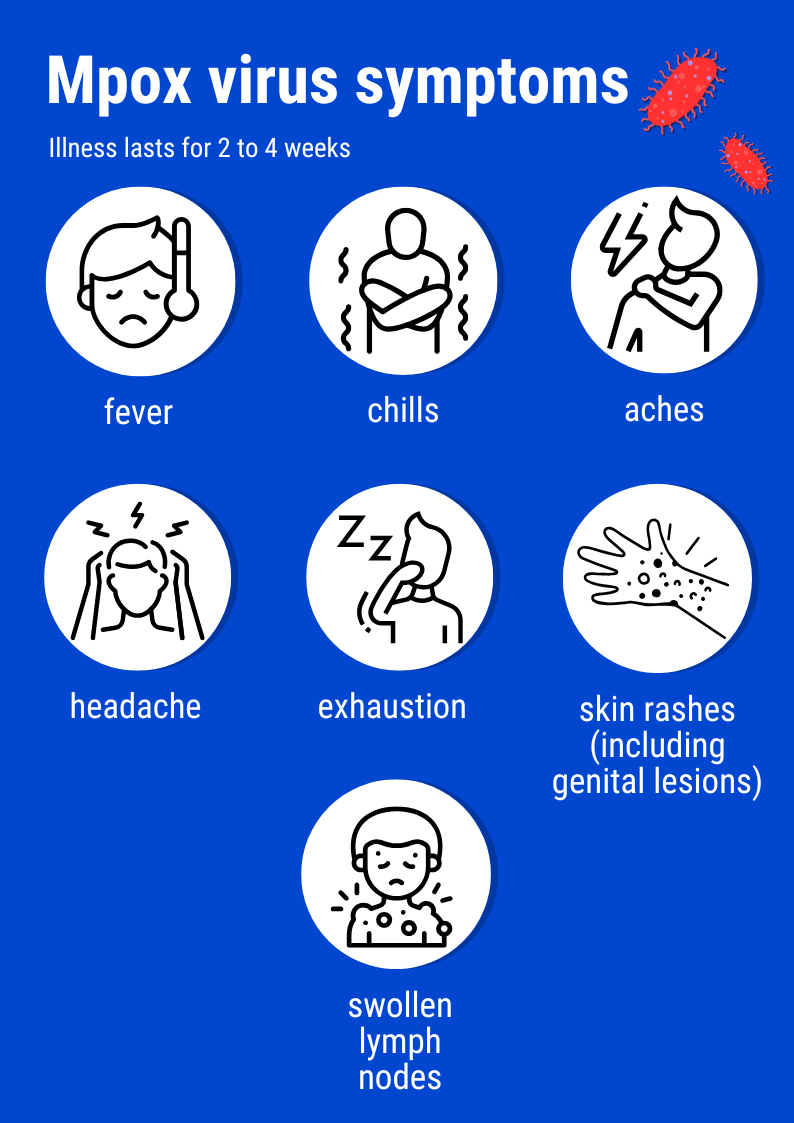
The fatality rate is much lower than for smallpox, but mpox is debilitating and can lead to death in vulnerable patients, especially in areas that are too far from medical infrastructure for any complications to be treated in time – these may include secondary skin infections, septicemia, encephalitis or respiratory difficulties. And fewer and fewer people are immunized against the virus. For many years, children systematically received the smallpox vaccine, until smallpox was declared to be eradicated in 1980. Studies show that the smallpox vaccine also offers 85% protection against mpox. But anyone born after 1980 will not have received the vaccine so is more likely to contract mpox and pass it on – and this cohort represents a growing proportion of the population.

A new strain of the virus
The 2022 outbreak seems a long time ago now, but mpox is still circulating. Hunters, including children, are continuing to be infected by animal reservoirs in endemic regions. And a new outbreak occurred at the start of 2024 in eastern DRC, this time transmitted by heterosexual contact. The outbreak began in a mining community and spread widely to neighboring countries. Some cases have already been exported – they can be identified because they are caused by a slightly different viral strain. Around 15 countries outside Africa, in Europe, Asia and America, detected the strain in the period between August 2024, when the World Health Organization (WHO) raised the alert, and March 2025 (1).
Emmanuel Nakouné, virologist at Institut Pasteur of Bangui, explains in 2 minutes the history of mpox and its recent discoveries © Institut Pasteur
In France, vigilance is still required: 215 cases of mpox were reported in 2024 (2). In particular the number of infections seems to be on the rise: 23 cases were reported in the first two months of 2025 alone (3). We are far from the nearly 5,000 cases that occurred during the peak of the 2022 outbreak, but this year's numbers may be higher than the 52 cases reported in 2023. Santé publique France points out that just 177,626 vaccine doses have been administered since 2022 (4), despite the fact that the population at risk is estimated to be 250,000 and two doses are needed for effective immunization.
Find out more: Monkeypox, an article by Antoine Gessain, Emmanuel Nakoune and Yazdan Yazdanpanah in the New England Journal of Medicine in 2022.
Travel and high-risk behavior
New fears were raised when the virus began showing up in other countries. A large outbreak in Nigeria in 2017 particularly set alarm bells ringing: for the first time, the virus was reported in built-up urban areas, especially Lagos. The emergence of a contagious infection in a densely populated area whose residents are more likely to travel represented a new level of risk. These fears were realized when the disease spread outside the African continent in 2022, causing outbreaks in a hundred countries, including France. In barely six months, the virus affected more than 84,000 people worldwide (5).
Why did the spark catch fire this time, even though the virus is still transmitted in the same way, through direct contact with skin lesions? "The virus began circulating among communities where it could spread more easily: men who have sex with men and have multiple partners, and sex workers," explains Antoine Gessain. "This became clear when a growing number of patients experienced skin lesions around the genital and anal regions and the mouth." These lesions can be itchy, and scratching them can cause secondary bacterial infections. And their position on the body can make them so painful that they are only bearable with powerful painkillers.
The epidemic was brought under control within a few months in most countries, thanks to a targeted information and vaccination campaign and a concerted effort from associations working with populations at risk. The fact that there was already a vaccine also played a part.
Afripox: an effective cooperative project
The Institut Pasteur in Paris and the Institut Pasteur de Bangui, in the CAR, launched a joint research project on mpox in 2019. The Afripox project has led to several discoveries. Scientists have identified how the disease emerged and evolved, and have pinpointed one of the most likely animal reservoirs. They are currently validating new rapid diagnostic tests in the laboratory and in the field, which will be useful for remote areas, and they have developed novel sequencing methods to monitor the different clades more effectively.
How climate change affects our bodies
With Darragh Duffy, Head of the Translational Immunology Unit, and Elizabeth Maloney, a postdoctoral fellow in the same unit
With temperatures on the rise around the world, we are seeing more frequent and intense heatwaves. What are the direct consequences for the human body? We take a closer look and fact-check some common theories.
A rise of 2°C is not a problem
FALSE France is heating faster than the global average. If the trend continues, its climate will be 2°C warmer in 2030 than it was at the beginning of the 20th century, 2.7°C warmer in 2050 and 4°C warmer in 2100. Since temperatures have already risen by 1.7°C (1), it is tempting to think that this won't make much of a difference. But we mustn't forget that with any additional rise, heatwaves – which already kill more than 176,000 people every year in Europe (2), – will be more frequent, longer and more intense. Days with temperatures over 40°C will become the norm, as will "tropical nights," when the temperature does not fall below 20°C. With no respite, the body's thermoregulatory mechanisms can soon come under strain. The central nervous system shows signs of distress, such as headache, dizziness, nausea, drowsiness and fainting, when it reaches temperatures higher than 41-42°C. If hyperthermia continues, it can lead to organ failure, with life-threatening consequences.
Only elderly people are affected
FALSE. Most visits to hospital emergency departments and heat-related deaths are in people over the age of 75, closely followed by young children, pregnant women and people weakened by disease. But as heatwaves become more intense, they are affecting a greater proportion of the population, including young people in good health. Several other groups are particularly vulnerable, especially people on low incomes, who cannot afford to live in well-insulated homes, and women. In women, rising temperatures can lead to more frequent and severe hot flushes as their internal cooling system kicks in more quickly, wearing the body out. In the 2003 heatwave, excess mortality was 15% higher in women than men in those over the age of 55.(4) People who work outdoors are also more affected, especially if their working hours are not adjusted during hot spells.
A spell of hot weather can lift your spirits
IT DEPENDS. If the temperature is 25°C, this is true. We can enjoy the good weather without any risk of feeling bad. But when it is very hot, the stifling conditions, intense thirst, tiredness at the slightest physical effort and difficulty concentrating are factors of stress and irritability. The mental health impact is also exacerbated by a lack of sleep during hot, sticky nights, or by eco-anxiety at this tangible manifestation of climate change.
Heat affects the immune system
TRUE. As fever is an effective defense mechanism against several diseases, we might think that an increase in atmospheric temperatures would be good for us and boost our lymphocytes. But this is not the case. A study recently demonstrated that climate change and the risks associated with it increase the severity of infections by 58% (4). This is partly because it puts greater pressure on the body's metabolic resources, since fever and fighting against hyperthermia both consume a great deal of energy and oxygen; and also because it causes stress, which has long been known to weaken the immune system, and because of other mechanisms that are yet to be elucidated.
There is nothing we can do about it
FALSE. All the health impacts described above highlight the importance of tackling climate change to limit the rise in temperatures as much as possible, and also point to the need for adaptation strategies at national and regional level (5).
Reminder of the sources used :
- Read the article on the climate of the futur Météo France, 2025 (in French)
- Read the review ONU, 2024 (in French)
Pesticides encourage the emergence of resistance in fungi
Interview with Sarah Dellière, a scientist in the Immunology of Fungal Infections Unit
Until recently, fungal infections mostly affected patients with weakened immune systems. But over the past few years, the few pathogenic fungi capable of infecting people with healthy immune systems have expanded their range, and others are becoming resistant to treatment.
Which people are likely to contract a severe fungal infection? And why are we seeing more infections now than we used to?
Sarah Dellière: Humans have always been surrounded by fungi. Yeasts are part of our microbiota and we are constantly breathing in mold. But our immune system either tolerates or destroys them. Immunocompromised people, such as individuals with HIV/AIDS, transplant patients and those with blood cancer, are more vulnerable. Physicians are aware of this and do their best to prevent opportunistic infections. But other cases are more difficult to anticipate. Some drugs used to treat cancer, autoimmune diseases and inflammatory disorders, although they do not suppress immunity completely, attack immune responses. And while these immune responses may have played a negative role in the disease being treated, they could have been useful in warding off pathogenic fungi. We only become aware of these effects once the drugs are already on the market. The situation is becoming even more problematic because at the same time we are seeing the emergence of treatment-resistant species.
Is this resistance linked to misuse of antifungal drugs, like antibiotic resistance in bacteria?
S. D.: This is probably the case for Trichophyton indotineae, which emerged in India around ten years ago and causes severe skin infections. But it is rare.
So why are we seeing resistance?
S. D.: There are not many effective drug families to treat fungal infections, so we use the same drugs in medicine and agriculture – especially azoles, which have broad spectrum activity. And therein lies the problem: when we spread them in fields, they don't just attack blight; they also attack fungi that are harmless for plants. Over time, resistance to azoles used in both agriculture and medicine can emerge. This was the case with Aspergillus fumigatus, a fungus which does not represent a threat to any crops but can attack the lungs of immunocompromised patients. Some strains of Aspergillus fumigatus have become resistant to first-line drugs, and there are no ideal alternatives since the other available treatments are toxic for humans.
Candida auris was first identified in 2009. This new species seems to have been selected because of excessive pesticide use, pollution and climate change. It is resistant to antifungal drugs, can withstand pollution and survives at temperatures over 40°C. It has become a major problem for hospitals in some world regions because it can take advantage of surgery or catheter insertion to infect the blood and trigger candidemia, the fungal version of septicemia.
So does climate change also play a part?
S. D.: Climate change leads to the selection of strains that can proliferate in warmer areas, meaning they can also tolerate the heat of the human body. It has also resulted in some species expanding their range. Coccidioides, which causes valley fever in patients with healthy immune systems, was historically only found in the arid regions of Arizona, but it has now been reported as far away as Canada.
Interview with Sarah Dellière, researcher in the Immunology of Fungal Infections unit. Credit: Institut Pasteur
Leishmaniasis is not just a dog disease
With: Gerald Spaeth, Head of Molecular Parasitology and Signaling Unit
Leishmaniasis is generally known in Europe as a canine disease, but it can also affect humans. Gerald Spaeth, Head of the Molecular Parasitology and Signaling Unit, introduces us to this parasitic illness transmitted by sandflies.
Trois formes cliniques
La leishmaniose cutanée, la plus fréquente, se caractérise par des lésions ulcérées sur les parties découvertes du corps. Ces lésions guérissent en général spontanément, mais peuvent laisser des cicatrices.
La leishmaniose cutanéomuqueuse, qui détruit les muqueuses de la bouche, du nez et de la gorge, ne guérit pas spontanément. Elle peut défigurer.
La leishmaniose viscérale se manifeste par une fièvre, une anémie, un amaigrissement, un gonflement du foie, de la rate et des ganglions lymphatiques. Aussi appelée kala-azar (« fièvre noire » en Hindi), elle est fatale dans 95 % des cas en l’absence de traitement.
Mode of transmission
Leishmaniasis is not contagious between humans, or even between dogs and their owners. It is contracted when an infected female sandfly bites a host to feed on their blood. The parasite is soon captured by the body's macrophages, but these immune cells, which normally eliminate microbes, are tricked. Instead of destroying the parasite, they host it and allow it to proliferate, eventually resulting in disease. Sandflies are infected when they feed on the blood of an infected animal or human and ingest contaminated macrophages.
Diagnosis and treatment
Diagnosis is based on a clinical examination and testing for parasites. If a patient is thought to have visceral leishmaniasis, a serological assay is also performed to confirm the diagnosis. Treatment, administered by injection or orally, lasts for several weeks. Sometimes it does not fully eliminate the parasite and in this case if the immune system is weakened the patient may relapse.
Risk factors
Impoverished populations are most affected. Unsanitary conditions, a lack of sanitation facilities and accumulated waste create breeding sites for sandflies. But infection does not necessarily result in disease. The risk of developing leishmaniasis after infection is higher in individuals with malnutrition or co-infections that weaken the immune system (e.g. HIV/AIDS).
Geographical distribution
Leishmania parasites are endemic in 99 countries, mainly in South-East Asia, South America and Africa.(1) Deforestation facilitates transmission by bringing humans closer to animal reservoirs, and climate change is encouraging the northward spread of sandflies. Cases of leishmaniasis have now been recorded in southern regions of France such as Languedoc, Provence, Côte d'Azur (or French Riviera) and Corsica.
Prevention
There is no vaccine or prophylactic treatment. In both humans and animals, only early treatment can limit the number of parasitic reservoirs and prevent the parasite from spreading.