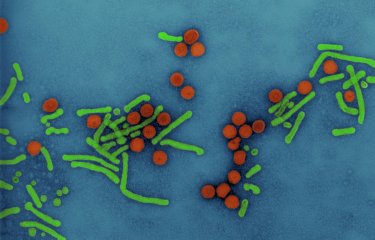
The Humoral Immunology Unit has identified neutralizing antibodies that are effective against the hepatitis E virus (HEV). They could be used for prevention or treatment against HEV infection, which affects approximately 20 million people worldwide every year. Structural analyses carried out with the NanoImaging Core Facility enabled atomic-scale visualization of the neutralization site recognized by the antibodies. The findings serve as a basis for the rational design of vaccines and immunotherapies against HEV.
Hepatitis E virus (HEV) infection is the leading cause of acute hepatitis, each year affecting approximately 20 million people worldwide and resulting in 30,000 to 40,000 deaths. Although HEV infection is usually self-limiting, it can develop into severe forms, including acute liver failure that may be fatal in pregnant women, or chronic hepatitis in immunocompromised individuals.
Antibodies targeting the capsid protein at the surface of the virus play a key role in controlling infection and offering protection, but their properties remain largely unknown. To date, there is no specific antiviral treatment against acute HEV infection, and although there is an effective preventive vaccine, it is only licensed in China and mainly recommended as an outbreak response measure.
In a study coordinated by the Humoral Immunology Unit led by Hugo Mouquet, scientists performed a detailed characterization of more than a hundred human monoclonal antibodies specific to the HEV capsid protein (CA), which they produced from the memory B cells of individuals in remission after exposure to the virus. Most of the antibodies recognized the different HEV variants and also interacted with rat hepatitis E virus, a significant zoonotic threat for humans.
Among the antibodies characterized, the scientists identified potent neutralizing antibodies that bind to the P (protruding) domain of the CA protein. They used cryo-electron microscopy to perform structural analyses of the CA protein in complex with three of these broadly neutralizing antibodies. The analyses, conducted in collaboration with Eduard Baquero Salazar from the NanoImaging Core Facility, enabled atomic-scale identification of the neutralization site recognized by the antibodies, situated on two P domain loops at the apex of the viral spike.
The results provide valuable insights into the protective humoral response to HEV and offer a solid basis for the rational design of HEV vaccines and immunotherapies. The neutralizing antibodies represent promising candidates for prophylactic and/or therapeutic treatment of HEV infection. In particular, long-acting versions of these antibodies, engineered to have an extended half-life, could provide protective immunity for immunocompromised populations and pregnant women.
These findings were published in May 2025 in the journal Science Advances.
References :
Structural Basis for Hepatitis E Virus Neutralization by Potent Human Antibodies. Luis M. Molinos-Albert, Eduard Baquero, Cyril Planchais, Virginie Doceul, Hicham El Costa, Estelle Mottez, Vincent Mallet, Stanislas Pol, Matthew L. Albert, Nicole Pavio, Cécile Alanio, Jordan D. Dimitrov, Hugo Mouquet. Sci Adv. 2025 May 9;11(19):eadu8811. doi: 10.1126/sciadv.adu8811. Epub 2025 May 7. PMID: 40333967