In 1965, the discovery of a mechanism known as allostery revolutionized our understanding of regulation in molecular biology. Sixty years on, we look back at a scientific and human journey that ushered in a new era of biochemistry and inspired huge swathes of research being conducted today.
In 1965, Jacques Monod, Jeffries Wyman and Jean-Pierre Changeux published an article(1) that would transform molecular biology. It described their discovery of the allosteric model.
- Jacques Monod was a French biochemist at the Institut Pasteur in Paris, who would receive the Nobel Prize in Physiology or Medicine that same year (along with François Jacob and André Lwoff) for other research on the regulation of gene expression in enzyme and virus synthesis.
- Jeffries Wyman was an American molecular biologist and biophysicist known for his research into proteins, amino acids and the physical chemistry of hemoglobin.
- Jean-Pierre Changeuxmeanwhile, was a young PhD student in Jacques Monod's laboratory. It was he who decided to focus on the regulation of protein activity as his PhD topic.
Sixty years after their discovery, the concept of allostery continues to drive therapeutic innovations and new ideas.
Jean-Pierre Changeux is now one of France's leading scientists, a neurobiologist and Professor Emeritus at the Institut Pasteur and the Collège de France. His research has had a significant impact on molecular biology and neuroscience, especially his work on brain receptors, synaptic selection and access to consciousness. He is also passionate about scientific outreach and has authored several mainstream science books, including:
- L’Homme neuronal (1983),
- Ce qui nous fait penser : la Nature et la Règle (avec Paul Ricoeur) (1998),
- and La Beauté dans le cerveau (2016).
Jean-Pierre Changeux sat down with Maurizio Brunori from the Accademia Nazionale dei Lincei, who organized an anniversary conference on allostery in Rome in May 2025, to talk to us about his discovery of allostery. Maurizio Brunori, Professor Emeritus at Sapienza University of Rome, is also recognized for his significant contribution to protein biochemistry and biophysics, with discoveries in the fields of protein structural dynamics, folding, function and evolution.
We delve into the fascinating scientific story of allostery and how it has helped us understand regulation in molecular biology.
Can you explain what allostery is?
Jean-Pierre Changeux : The theory of allostery mainly applies to proteins, large molecules composed of long chains of smaller amino acids. Allostery describes the phenomenon whereby small molecules known as ligands bind to certain regulatory proteins at a site that is topologically separate from the active site, causing a conformational change in the protein which regulates the activity of the active site at a distance. This property enables precise, rapid regulation of the major functions of living beings, playing a key role in bacteria, red blood cells and neurons.
Allostery gives living cells a powerful capacity for adaptation and evolution. It is not just a minor detail of living systems but a fundamental property of molecules that controls cellular functions.

This discovery must have been an exciting moment for you working in the laboratory. Tell us more.
J-P. C. : It all stemmed from a desire to understand enzyme regulation at the molecular level based on the experimental data in my PhD thesis. The main finding of my thesis was that regulation by chemical signals is complemented by "cooperativity," a property whereby the binding of a ligand to its site strengthens ligand binding to another identical site on the protein. This results in sigmoidal response curves, rather than the usual hyperbolic curves. The scientific discovery was that these two properties can be abolished simultaneously, signaling a general reorganization of the protein. We sought to model this transition and describe it mathematically.
How would you summarize the model you proposed to describe protein states?
J-P. C. : Our theory was that an allosteric protein can exist in equilibrium in several states, and that interaction with a ligand causes it to switch from one state to another, modifying both protein activity and cooperative effects. This two-state model that we proposed with Jacques Monod and Jeffries Wyman, known as the MWC (Monod-Wyman-Changeux) model, led to a paradigm shift in our conception of proteins, starting with hemoglobin and extending to a whole family of regulatory proteins.
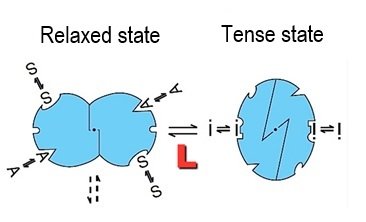
Maurizio Brunori : I feel so lucky to have witnessed the discovery of allostery. The concept emerged thanks to the combined efforts of a group of accomplished, passionate scientists. Over and above their individual talents, Wyman, Monod and Changeux formed a trio who shared the same ambition and energy and were linked by a unique intellectual connection. We wanted to pay tribute to this creative and remarkably successful scientific collaboration in May 2025 in Rome, by looking back at the impact of the discovery.
Yes – what are the implications of this discovery today? It is referred to as a paradigm shift in biochemistry.
J-P. C. : The allosteric model radically changed our conception of protein biology. It gave us an understanding of :
- regulatory signal transduction: the mechanism by which a cell responds to the information it receives;
- cooperativity between different sites: a biochemical property that reflects cooperation between several substrate-binding sites; the binding of a substrate to one site facilitates or hinders the binding of the substrate to another site;
- conformational plasticity: the protein's ability to adopt several distinct, interconvertible spatial arrangements with distinct activities;
- selection by the regulatory signal of specific conformational states which are now known at the atomic level.
Together, these properties give living beings a considerable selective advantage. In particular, they contribute to the constancy of the internal environment described by Claude Bernard and the cybernetic regulation of living organisms proposed by Norbert Wiener.
Allostery has also paved the way for modeling and for the rational design of new drugs that act on receptors or enzymes. For the past twenty years, the scientific community has been exploring the functional diversity of allosteric proteins with a view to developing new treatments.
M. B. : Allostery has revolutionized our view of biology. It has given us a fundamental, molecular-level understanding of how the complexity of living organisms is organized and controlled. For me, two aspects are essential: first, allostery provides a genuine basis for thermodynamics applied to living organisms, enabling equilibria to be regulated on the basis of different parameters. Second, it illustrates Darwinian selection at the molecular level: the "choice" between various molecular conformations explains many of life's emerging properties. This dual aspect – thermodynamic and evolutionary – has opened up a raft of new possibilities in fundamental biochemistry, leading to extensive research on membrane proteins and neuronal receptors.
How is the discovery of allostery continuing to inspire researchers, especially those researching diseases?
J.-P. C. : Allostery permeates many areas of biomedical research, particularly neuroscience, where it helped elucidate the mechanisms of neurotransmitter receptors, including the acetylcholine receptor. Mutations in genes coding for allosteric proteins are responsible for multiple diseases, especially neuropsychiatric disorders, as well as resistance to infectious agents. For the proteins concerned, these mutations may result in either a loss of function or a "gain of function." To develop new therapeutic agents, knowledge of the genome is not enough; precise identification of the allosteric phenotype of the protein concerned is also required. Precision medicine is now complemented by a new "precision pharmacology."
- Jacque Monod, Jeffries Wyman, Jean-Pierre Changeux, On the nature of allosteric transitions: A plausible model, Journal of Molecular Biology, Volume 12, Issue 1, 1965, Pages 88-118, ISSN 0022-2836, DOI : 10.1016/S0022-2836(65)80285-6.
- Brunori, M. Wandering about allostery. Biol Direct 19, 64 (2024). DOI : 10.1186/s13062-024-00502-0
- Marco Cecchini, Pierre-Jean Corringer, Jean-Pierre Changeux, Vol. 93:339-366 (Volume publication date August 2024). DOI : 10.1146/annurev-biochem-030122-033116
- Allostery turns 60 | Accademia Nazionale dei Lincei, Rome, celebration meeting on May 20th 2025
- The beauty and the splendor of the truth: interviews on autobiographical recollections, Jean-Pierre Changeux, François L'Yonnet