Cocaine addiction is a chronic disorder with a high rate of relapse for which no effective treatment is currently available. Scientists from the Institut Pasteur, the CNRS, Inserm and the Paris Public Hospital Network (AP-HP) recently demonstrated that two gene mutations involved in the conformation of nicotinic receptors in the brain appear to play a role in various aspects of cocaine addiction. The results of the study were published in Progress in Neurobiology.
There are approximately 18 million users worldwide, and cocaine is implicated in more than 50% of overdose deaths in the United States and 25% in France. It is also one of the only drugs for which there is no approved pharmacological treatment.
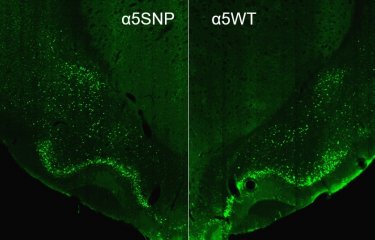
Cocaine acts primarily in the brain by blocking the dopamine transporter, thereby increasing the concentration of this "pleasure" molecule in the reward system. But cocaine can also act directly on the nicotinic receptors1 in the brain. Several human genetics studies have recently suggested that a mutation in the gene encoding the α5 subunit of nicotinic receptors, hereafter referred to as ‘α5SNP’, already known to increase the risk of tobacco dependence,2 may conversely also confer "protection" against cocaine addiction. This mutation is highly present in the general population (approximately 37% of Europeans and up to 43% of the Middle Eastern population carry it), so it is important to determine how it affects cocaine addiction and, more generally, to better understand the role of the α5 nicotinic subunit in the effects of cocaine.
Scientists of the Integrative Neurobiology of Cholinergic Systems Unit (Institut Pasteur/CNRS) began by evaluating the role of the α5 nicotinic subunit and the impact of the α5SNP mutation on various processes involved in the development of cocaine addiction in animal models. The results obtained were then used to characterize more specifically its impact on humans.
The scientists observed that the α5SNP mutation reduces the voluntary intake of cocaine upon first exposures. "These preclinical data suggest that the mutation protects against cocaine addiction by modulating an early phase in the addiction cycle," comments Morgane Besson, one of the lead authors of the study. Working in collaboration with the Paris Public Hospital Network (AP-HP) and Inserm, the scientists then confirmed this significant effect in approximately 350 patients with cocaine addiction: those with the mutation exhibited a slower transition from first cocaine use to the emergence of signs of addiction. At the same time, the authors showed that a total absence of the α5 nicotinic subunit increased the risk of relapse after withdrawal in preclinical models. This led the scientists to identify another mutation in another nicotinic subunit, β4, associated with a shorter time to relapse after withdrawal in addicted patients.
Taken together, these results elucidate the role played by both a highly frequent mutation in the α5 nicotinic subunit and the subunit itself in various stages of cocaine addiction. The research suggests that drugs modulating nicotinic receptors containing this α5 subunit could represent a novel therapeutic strategy for cocaine addiction.
[1] Nicotinic receptors are located in the cell membrane and respond to the neurotransmitter acetylcholine. They act like pores for communication between the cell's internal and external environment. Nicotinic receptors are important modulators of various functions in the central nervous system. Nicotine is an agonist for the receptors, meaning that it can act on these targets instead of acetylcholine.
[2] Smoking cessation: a genetic mutation involved in relapse
Source :
Alterations in nicotinic receptor alpha5 subunit gene differentially impact early and later stages of cocaine addiction: A translational study in transgenic rats and patients, Progress in Neurobiology, 22 août 2020
Benoit Forgeta, Romain Icicka,bc,d, Jonathan Roberta, Caroline Correiaa, Marie S. Prevoste, Marc Gielend,e, Pierre-Jean Corringere, Frank Bellivierb,d,e, Florence Vorspanb,c,d, Morgane Bessona,*, Uwe Maskosa,*
a Neurobiologie Intégrative des Systèmes Cholinergiques, CNRS UMR3571, Institut Pasteur, 25 rue du Dr Roux, 75724, Paris Cedex 15, France
b Département de Psychiatrie et de Médecine Addictologique, Groupe Hospitalier Saint-Louis – Lariboisière – Fernand Widal, Assistance-Publique Hôpitaux de Paris, 75010, Paris, France
c INSERM UMR_S1144, 4 avenue de l’Observatoire, 75006, Paris, France
d Université Sorbonne – Paris – Cité, Paris, France
e Unité Récepteurs-Canaux, CNRS UMR3571, Institut Pasteur, 25 rue du Dr Roux, 75724 Paris Cedex 15, France
*equal contribution