Although the different SARS-CoV-2 variants currently in circulation are undoubtedly less severe in vaccinated individuals in the general population, immunocompromised people are at greater risk of developing severe forms of COVID-19. Monoclonal antibodies currently offer the best approach, both as a preventive and curative treatment for these patients. Scientists from the Institut Pasteur and Inserm identified two potent neutralizing antibodies from convalescent COVID-19 individuals that proved effective against SARS-CoV-2 variants of concern. These human antibodies are prime candidates for the development of immunotherapies to prevent severe forms and/or treat COVID-19. The findings were published in The Journal of Experimental Medicine on June 15, 2022.
Antibody and memory B cell responses to SARS-CoV-2 spike (S) proteins contribute to long-term immune protection against severe forms of COVID-19. Immunotherapy based on neutralizing antibodies can also prevent severe forms in individuals who fail to respond to vaccination, such as immunocompromised patients, who number approximately 230,000 people in France. The clinical benefits of anti-SARS-CoV-2 "monoclonal"[1] antibodies have already been established in clinical trials to treat COVID-19 patients and prevent the onset of severe forms.
In this study conducted by scientists in the Institut Pasteur's Humoral Immunology laboratory led by Dr. Hugo Mouquet (a joint Inserm unit), in collaboration with several Institut Pasteur and Inserm teams, the immune response to SARS-CoV-2 in convalescent COVID-19 individuals was investigated through detailed analysis of antibodies targeting the SARS-CoV-2 S protein at serological level (antibodies in the bloodstream), cellular level (antibody-producing B cells) and molecular level (monoclonal antibodies). In particular, detailed characterization of a hundred human monoclonal antibodies specific to the SARS-CoV-2 S protein, cloned from memory B cells isolated from convalescents, revealed the diversity of their antiviral functions, such as the neutralization or destruction of infected cells.
"Two of the potent neutralizing antibodies identified, Cv2.1169 and Cv2.3194, are broad-spectrum antibodies meaning that they are effective against SARS-CoV-2 variants of concern: Alpha, Beta, Gamma, Delta and Omicron BA.1 and BA.2. The monoclonal antibody Cv2.1169, tested in animal models of SARS-CoV-2 infection, demonstrated prophylactic (preventive) and therapeutic activity in vivo," commented Hugo Mouquet, Head of the Institut Pasteur's Humoral Immunology laboratory (a joint Inserm unit).
Since the Cv2.1169 antibody was isolated from a mucosa-derived B cell, this type of antibodies in the mucosa of convalescent individuals could help protect them from infection with SARS-CoV-2 variants.
"These potent human broadly neutralizing monoclonal antibodies are promising candidates for the development of immunotherapies in humans to prevent and/or treat COVID-19," added Hugo Mouquet.
The Institut Pasteur has filed an international patent application to protect the neutralizing antibodies identified in this study ["Human neutralizing monoclonal antibodies against SARS-CoV-2 and their use thereof" (PCT/EP2022/058777)]. This patent application is the subject of an exclusive worldwide license agreement with SpikImm – a biotech established by Truffle Capital and the Institut Pasteur – which develops the antibodies as easy-to-administer (by intramuscular injection), long-acting antibodies for preventing COVID-19 (pre-exposure prophylaxis) in immunocompromised patients who often exhibit a weak or no immune response after full vaccination. SpikImm plans to start clinical trials in July 2022. The French National Ad Hoc Steering Committee for Therapeutic Trials and Other Research (CAPNET) recently awarded this Phase I trial the "National Research Priority" label.
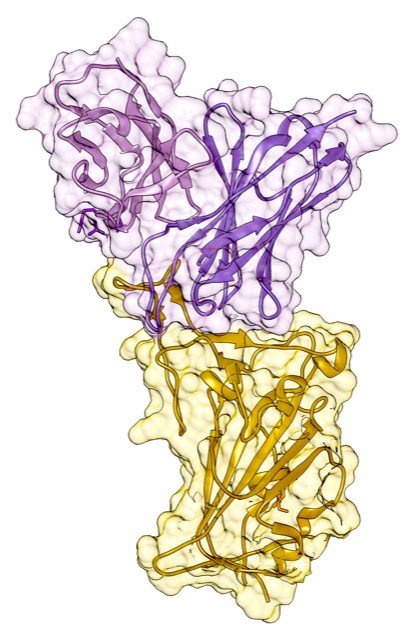
Structure of the variable domains of the Cv2.1169 antibody (purple) in complex with the S protein domain involved in SARS-CoV-2 receptor binding (receptor binding domain or RBD in yellow), obtained by X-ray crystallography in the Structural Virology laboratory (Institut Pasteur). © Hugo Mouquet, Institut Pasteur
Source
Potent Human Broadly SARS-CoV-2 Neutralizing IgA and IgG Antibodies Effective Against Omicron BA.1 and BA.2, Journal of Expertimental Medicine, June 15, 2022
Cyril Planchais1,2, Ignacio Fernández3,4, Timothée Bruel4,5,18, Guilherme Dias de Melo6,18, Matthieu Prot7,18, Maxime Beretta1,2, Pablo Guardado-Calvo3,4, Jérémy Dufloo4,5, Luis M. Molinos-Albert1,2, Marija Backovic3,4, Jeanne Chiaravalli8, Emilie Giraud8, Benjamin Vesin9,10, Laurine Conquet11, Ludivine Grzelak4,5, Delphine Planas4,5, Isabelle Staropoli4,5, Florence Guivel-Benhassine4,5, Thierry Hieu12, Mikaël Boullé8, Minerva Cervantes-Gonzalez13, Marie-Noëlle Ungeheuer14, Pierre Charneau9,10, Sylvie van der Werf4,15,16, Fabrice Agou8, French COVID Cohort Study Group#, CORSER Study Group#, Jordan D. Dimitrov17, Etienne Simon-Lorière7,19, Hervé Bourhy6,19, Xavier Montagutelli11,19, Félix A. Rey3,4,19, Olivier Schwartz4,5,19, Hugo Mouquet1,2,20
1Institut Pasteur, Université Paris Cité, Laboratory of Humoral Immunology, F-75015 Paris, France
2INSERM U1222, F-75015 Paris, France
3Institut Pasteur, Université Paris Cité, Structural Virology Unit, F-75015 Paris, France
4CNRS UMR3569, F-75015 Paris, France
5Institut Pasteur, Université Paris Cité, Virus & Immunity Unit, F-75015 Paris, France
6Institut Pasteur, Université Paris Cité, Lyssavirus Epidemiology and Neuropathology Unit, F-75015 Paris, France
7Institut Pasteur, Université Paris Cité, G5 Evolutionary Genomics of RNA Viruses, F-75015 Paris, France
8Institut Pasteur, Université Paris Cité, Chemogenomic and Biological Screening Core Facility, C2RT, F-75015 Paris, France
9Pasteur-TheraVectys, F-75015 Paris, France
10Institut Pasteur, Université Paris Cité, Molecular Virology & Vaccinology Unit, F-75015 Paris, France
11Institut Pasteur, Université Paris Cité, Mouse Genetics Laboratory, F-75015 Paris, France
12 Institut Pasteur, Université Paris Cité, Functional Genetics of Infectious Diseases Unit, F-75015 Paris, France
13Department of Epidemiology, Biostatistics and Clinical Research, Assistance Publique-Hôpitaux de Paris, Bichat Claude Bernard University Hospital, INSERM CIC-EC 1425, Paris, France
14Institut Pasteur, Université Paris Cité, Investigation Clinique et Accès aux Ressources Biologiques (ICAReB), Center for Translational Research, F-75015 Paris, France
15Institut Pasteur, Université Paris Cité, Molecular Genetics of RNA Viruses, F-75015 Paris, France
16Université de Paris, Paris, France
17Centre de Recherche des Cordeliers, INSERM, Sorbonne Université, Université de Paris, 75006 Paris, France
18Equal contribution.
19These senior authors contributed equally.
20Lead contact.