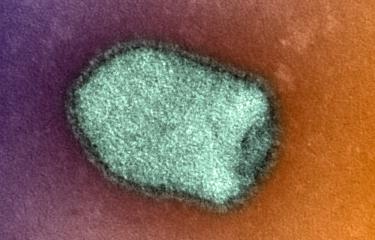
Rabies virus kills over 50,000 people each year. Although current rabies vaccines are effective, they do not offer lifelong protection and booster doses are required. Institut Pasteur scientists, in collaboration with researchers from La Jolla Institute for Immunology (LJI) in the United States, captured new high-resolution views of rabies virus, revealing potential vaccine targets. These findings were published in the journal Science Advances on June 17, 2022.
Rabies virus kills over 50,000 people each year, many of them children. Some victims, especially kids, don't realize they've been exposed until it is too late. For others, post-exposure prophylaxis is either unavailable or inaccessible, since its average cost is too high for exposed communities in developing countries.
Rabies vaccines come with one major drawback: they do not offer lifelong protection. Pets require a booster dose every 1 to 3 years. Rabies vaccines for humans and domestic animals are currently made from killed virus. But this inactivation process can cause the molecules to become misshapen – so these vaccines aren’t showing the right form to the immune system.
In collaboration with an LJI team, scientists from the Institut Pasteur's Lyssavirus Epidemiology and Neuropathology Unit may have discovered the path to better vaccine design. In a new study, published in Science Advances, the scientists share one of the first high-resolution looks at the rabies virus glycoprotein in its vulnerable "trimeric" form.
"If we made a better shaped, better structured vaccine, would immunity last longer?" asks the study's last author, LJI Professor, President & CEO Erica Ollmann Saphire, Ph.D. "The rabies glycoprotein is the only protein that rabies virus expresses on its surface, which means it is going to be the major target of neutralizing antibodies during an infection," says LJI Postdoctoral Fellow Heather Callaway, Ph.D., who serves as the study's first author.
"Rabies is the most lethal virus we know. It is so much a part of our history – we’ve lived with its specter for hundreds of years," adds Saphire. "Yet scientists have never observed the organization of its surface molecule. It is important to understand that structure to make more effective vaccines and treatments – and to understand how rabies and other viruses like it enter cells."
"This understanding is crucial to design better vaccines and to develop treatments to cure rabid patients," adds Prof. Hervé Bourhy, head of the Institut Pasteur's Lyssavirus Epidemiology and Neuropathology Unit.
The rabies virus glycoprotein: a multifaceted protein
Scientists don't know exactly why rabies vaccines don't provide long-term protection, but they do know that its shape-shifting proteins are a problem.
Like a Swiss Army knife, the rabies glycoprotein has sequences that unfold and flip upward when needed. The glycoprotein can shift back and forth between pre-fusion (before fusing with a host cell) and post-fusion forms. It can also fall apart, changing from a trimer structure (where three copies come together in a bundle) to a monomer (one copy by itself).
This shapeshifting gives rabies a kind of invisibility cloak. Human antibodies are built to recognize a single site on a protein. They can't follow along when a protein transforms to hide or move those sites.
The new study gives scientists a critical picture of the correct glycoprotein form to target for effective, long-term antibody protection.
Capturing the glycoprotein at last
Over the course of three years, the scientists worked to stabilize and freeze the rabies glycoprotein in its trimeric form. This "pre-fusion" form is the shape the glycoprotein takes before it infects human cells.
The teams paired the glycoprotein with a human antibody, which helped them pinpoint one site where the viral structure is vulnerable to antibody attacks. The scientists then captured a 3D image of the glycoprotein using cutting-edge cryo-electron microscope equipment at LJI. "This helps us to understand the mode of action of the antibodies neutralizing the virus," says Institut Pasteur's Hervé Bourhy.
The new 3D structure highlights several key features scientists hadn't seen before. Importantly, the structure shows two key pieces of the virus structure, called the fusion peptides, the way they appear in real life. These two sequences link the bottom of the glycoprotein to the viral membrane, but project into the target cell during infection. It is very hard to get a stable image of these sequences.
The scientists solved this problem by capturing the rabies glycoprotein in detergent molecules. That enabled them to see how the fusion sequences are attached before they snap upward during infection.
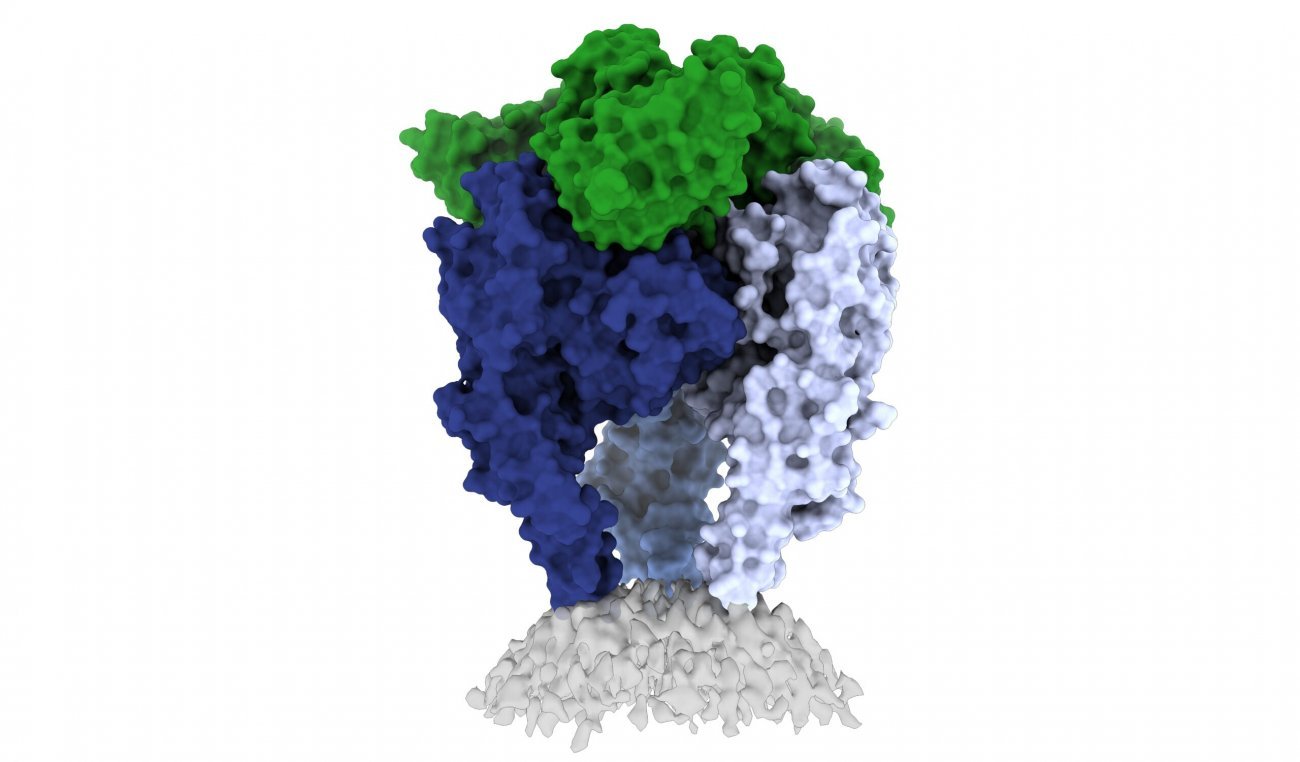 |
|
Scientists from La Jolla Institute for Immunology (LJI) and the Institut Pasteur have shed light on the structure of the rabies virus glycoprotein, seen here (image courtesy of Heather Callaway, Ph.D., LJI) |
Now that scientists have a clear view of this viral structure, they can better design vaccines that tell the body how to make antibodies to target the virus.
Instead of being exposed to four-plus different protein shapes, the immune system should really just see one – the right one. This could lead to a better vaccine.
Preventing a family of viruses
Broader immunity could help people in regular contact with animals, such as veterinarians and wildlife workers, as well as the billions of people who may accidentally come in contact with a rabid animal. Rabies is endemic across every continent except Antarctica and infects numerous species including dogs, racoons, bats, and skunks.
This new work may also open the door to a vaccine to protect against the whole Lyssavirus genus, which includes rabies and similar viruses that can spread to humans from mammals other than dogs.
Scientists at the LJI Center for Infectious Disease and Vaccine Research and the Institut Pasteur's Lyssavirus Epidemiology and Neuropathology Unit are continuing their research and are now seeking to capture more images of rabies virus and its relatives together with neutralizing antibodies. Because these structures of the rabies virus in this conformational state had not previously been available, it was hard to design a broad-spectrum vaccine. Scientists are working on solving several of these structures, which could reveal antibody targets that lyssaviruses have in common.
Source
Structure of the rabies virus glycoprotein trimer bound to a prefusion-specific neutralizing antibody, Science Advances, 17th June 2022
Heather M. Callaway1, Dawid Zyla1, Florence Larrous2, Guilherme Dias de Melo2,
Kathryn M. Hastie1, Ruben Diaz Avalos1, Alyssa Agarwal1, Davide Corti3,
Hervé Bourhy2, Erica Ollmann Saphire1
1 Center for Infectious Disease and Vaccine Research, La Jolla Institute for Immunology, La Jolla, CA, USA.
2 Institut Pasteur, Université Paris Cité, Unit of Lyssavirus Epidemiology and Neuropathology, World Health Organization Collaborating Centre for Reference and Research on Rabies, Paris, France.
3 Humabs Biomed SA, a subsidiary of Vir Biotechnology, Bellinzona, Switzerland.
4 Department of Medicine,
University of California San Diego, La Jolla, CA, USA.
*Corresponding author.