Bordetella pertussis is the agent responsible for whooping cough, a disease that is currently on the rise. In particular, the bacterium produces the CyaA toxin. A consortium including several teams from the Institut Pasteur, CNRS, and Sorbonne University, working closely with the C2RT Proteins Pole technical cores and several SOLEIL and ESRF synchrotron light lines, has examined the entry mechanism of the CyaA toxin in target cells. This work has revealed a membrane translocation mechanism that has proved very useful in terms of knowledge of living biology.
The bacterium Bordetella pertussis causes whooping cough, an increasingly prevalent contagious disease that can be particularly severe, or even fatal, for infants and vulnerable individuals. Bordetella pertussis produces numerous virulence factors including the protein CyaA, a toxin that contributes to the early stages of bacterial colonization of infected individuals’ lungs. In particular, it intoxicates innate immune cells, especially alveolar macrophages, which defend against pathogens in the alveoli (air sacs) in our lungs.
Membrane translocation of CyaA, a key step in the cell intoxication process
The CyaA toxin uses an original mechanism to penetrate the interior of our cells: first, it binds to a specific receptor on the surface of target cells, and then, part of the polypeptide chain, the catalytic domain, is directly transported through the plasma membrane to the target cell cytosol through a process known as membrane translocation. The CyaA catalytic domain is subsequently activated by a cellular protein (known as calmodulin) to synthesize cAMP, a molecule essential for regulating cell physiology. However, the large quantities of cAMP generated by CyaA prompt deterioration of cell physiology, and in particular, inhibition of innate immune cells’ phagocytic functions, thus giving the bacterium free rein to colonize the lungs.
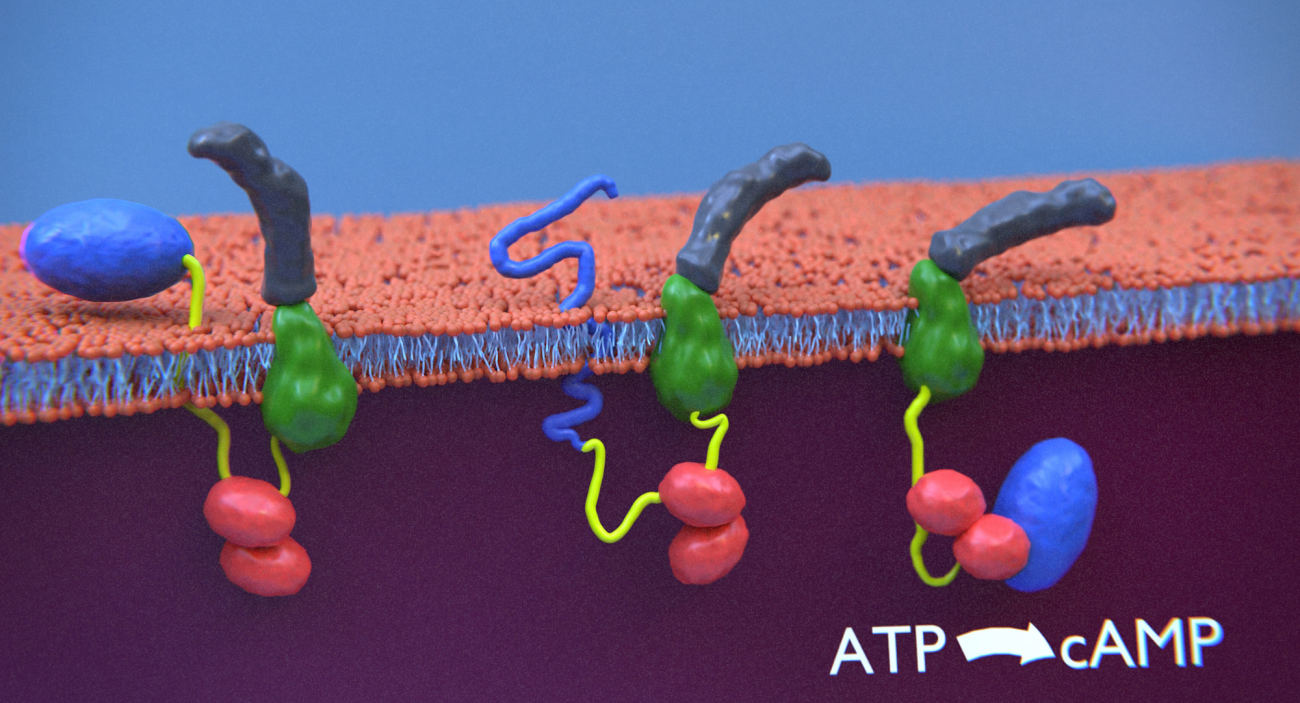
The mechanism of cell invasion by bacterial toxins is still poorly understood. The CyaA toxin from Bordetella pertussis, the causative agent of whooping cough, directly translocates its catalytic domain across plasma membrane. The membrane-permeabilizing segment (yellow) of CyaA translocates across the plasma membrane and binds calmodulin (red), which assists the entry of the catalytic domain (blue) into host cells while the hydrophobic and acylation domains (green) interact with the membrane and the C-terminal Repeat-in-Toxin domain (grey) remains in the extra-cellular milieu.
credit: Julien Husson https://cellmechanics.jimdofree.com/
CyaA membrane translocation utilizes molecular harpooning
Although we have been aware for some time that calmodulin activates the CyaA catalytic domain, the membrane translocation mechanism has remained an enigma. "We have demonstrated that a CyaA region known as the translocation region or TR is able to cross the plasma membrane of target cells in a similar way to antibiotic peptides. We have also demonstrated that this TR region interacts with calmodulin with very high affinity. The TR region "harpoons" the calmodulin in the cytosol, an interaction that traps the TR region in the cytosol and forces transport of the catalytic domain through the membrane," explains Alexandre Chenal, Head of the BiophysiCyaA Group in the Biochemistry of Macromolecular Interactions Unit at the Institut Pasteur. The high affinity between the TR region and calmodulin enables this transport of the catalytic domain from the cell exterior to its interior. The catalytic domain unfolds easily on the cell surface, encouraging its passage through the plasma membrane, pulled by the complex formed by the CyaA TR region and calmodulin in the cytosol.
This study enables greater understanding of the protein membrane translocation mechanism in general, and at molecular level, helps identify the residues involved in this process. Moreover, from a thermodynamic perspective, this study should enable us to quantify the energy (the tractive force, as it were) required to translocate and vectorize therapeutic biomolecules in target cells such as tumor cells.
Finally, this study illustrates synergies between research teams, C2RT platforms, particularly the Molecular Biophysics Platform, the Crystallography Platform, and the Biological NMR Technological Platform, the DISCO, SWING and PROXIMA lines (SOLEIL Synchrotron, St Aubin, France), and MASSIF lines (ESRF Synchrotron, Grenoble, France).
Source:
A High-Affinity Calmodulin-Binding Site in the CyaA Toxin Translocation Domain is Essential for Invasion of Eukaryotic Cells, Advanced Science, First published: 08 March 2021, https://doi.org/10.1002/advs.202003630
Alexis Voegele1,2#, Mirko Sadi1,2#, Darragh P O’Brien1#, Pauline Gehan3#, Dorothée Raoux-Barbot1, Maryline Davi1, Sylviane Hoos4, Sébastien Brûlé4, Bertrand Raynal4, Patrick Weber5, Ariel Mechaly5, Ahmed Haouz5, Nicolas Rodriguez3, Patrice Vachette6, Dominique Durand6, Sébastien Brier7, Daniel Ladant1*, Alexandre Chenal1*
1: Biochemistry of Macromolecular Interactions Unit, Department of Structural Biology and Chemistry, Institut Pasteur, CNRS UMR3528, 75015 Paris, France
2: Université de Paris, Sorbonne Paris Cité, Paris, France
3: Sorbonne University, École normale supérieure, PSL University, CNRS, Biomolecules Laboratory, LBM, 75005 Paris, France
4: Molecular Biophysics Platform, Institut Pasteur, UMR 3528 CNRS, Paris, France
5: Institut Pasteur, Crystallography-C2RT Platform, UMR-3528 CNRS, Paris, France
6: Université Paris-Saclay, CEA, CNRS, Institute for Integrative Biology of the Cell (I2BC), 91198, Gif-sur-Yvette, France
7: Biological NMR Technological Platform, Center for Technological Resources and Research, Department of Structural Biology and Chemistry, Institut Pasteur, CNRS UMR3528, 75015 Paris, France