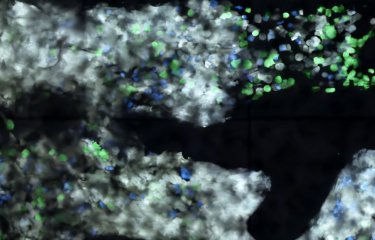
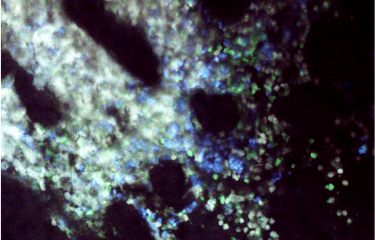
Among the characteristics defining our constituent organs are their different rigidities. Changes in these physical properties occur during tumor development. Tumors use the rigidity of their surrounding environment to facilitate their migration to neighboring tissue, which in turn facilitates tumor invasion and metastasis. Scientists at the Institut Pasteur have identified a new element enabling cells to "sense" and "respond" to substrate rigidity. This discovery paves the way for potential new therapies.
Cells in our bodies communicate among themselves through chemical molecules that they release and perceive in their environment. However, our organs are not solely defined by their biochemical properties. They are also defined by their physical properties, in particular the rigidity of their constituent tissue. In terms of rigidity, bones are high on the scale, muscles are less rigid, and the brain is very soft. These tissues' constituent cells are adapted to these physical properties which regulate their behavior and functions.
Mechanical properties that change over a lifespan
Tissue mechanics can change over our lifespan. This occurs during embryonic development and also in adults. For example, scars are often more rigid than the initial tissue. Some blood vessels may also become more rigid and less elastic. Such mechanical alterations to tissue cause changes in the behavior of its constituent and surrounding cells.
Increased rigidity involved in tumor development
Such changes appear to play a key role in cancer development. Tumors are indeed generally characterized by increased rigidity and can therefore sometimes be identified by palpation. How does tumor tissue rigidity affect the behavior of cancer cells? "Increased rigidity of the substrate [which surrounds the cells, ed.] modifies the behavior of cancer cells, facilitates cell migration, and contributes to tumor invasion. Cells of brain tumors such as glioblastomas then tend to migrate along blood vessels that are more rigid than the rest of the brain tissue," explains Sandrine Etienne-Manneville, head of the Institut Pasteur's Cell Polarity, Migration and Cancer Laboratory.
αTAT1 – an enzyme facilitating tumor cell migration
No explanation has previously been provided for cells’ adaptation mechanisms or ability to "sense" more rigid or softer elements. Sandrine Etienne-Manneville’s unit has revealed the role of microtubules or fibers forming the cytoskeleton that gives cells their structure. She explains: "These microtubules come close to places where the cell is in contact with its environment and are modified by an enzyme known as αTAT1 when substrates exhibit optimal rigidity." This modification of the microtubules causes an increase in the forces applied by the cell to its surrounding substrate, facilitating migration. By blocking αTAT1, it is possible to prevent microtubule modification, which means that cells are not able to adapt to the rigidity of their environment, thus impeding their migration.
These observations pave the way for potential new therapies. By modifying tissue rigidity or preventing cells from "sensing" their rigidity (e.g. by blocking αTAT1), it may be possible to block tumor invasion.
This study is part of the Cancer Initiative of the Institut Pasteur's strategic plan for 2019-2023.
Source:
Microtubules tune mechanosensitive cell responses, Nature materials, Date à venir
Shailaja Seetharaman1, 2, Benoit Vianay3, Vanessa Roca1, Aaron Farrugia4, Chiara De Pascalis1, Batiste Boëda1, Florent Dingli5, Damarys Loew5, Stéphane Vassilopoulos6, Alexander Bershadsky4, Manuel Théry3, Sandrine Etienne-Manneville1*
1 Cell Polarity, Migration and Cancer Unit, Institut Pasteur, UMR3691 CNRS, Equipe Labellisée Ligue Contre le Cancer, F-75015, Paris, France.
2 Université Paris Descartes, Sorbonne Paris Cité, 12 Rue de l'École de Médecine, 75006 Paris, France.
3 Paris University, INSERM, CEA, Hôpital Saint Louis, Institut Universitaire d’Hematologie, Paris, France.
4 Mechanobiology Institute, National University of Singapore, T-lab, 5A Engineering Drive 1, Singapore, 117411, Singapore.
5 Institut Curie, PSL Research University, Centre de Recherche, Laboratoire de Spectrométrie de Masse Protéomique, 26 rue d’Ulm, Paris 75248 Cedex 05, France.
6 Sorbonne Université, INSERM UMRS 974, Institute of Myology, Paris, France.