Scientists from the Institut Pasteur and the CNRS[1], in collaboration with Imperial College London and the University of Vienna, Austria, have identified antibodies that can efficiently neutralize both the dengue virus and the Zika virus. The description of the binding site for these antibodies on the viral envelope, identical for both viruses, could lead to the development of a universal vaccine that offers simultaneous protection against dengue and Zika virus disease. These results were published in the journal Nature on June 23, 2016.
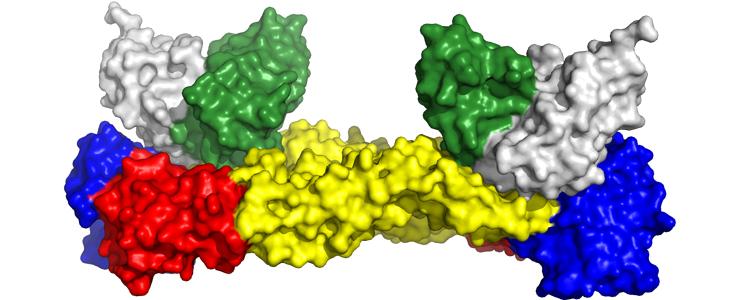
3D structure of the Zika virus envelope protein (red, yellow and blue) in complex with the neutralizing antibody (in green and white). © Institut Pasteur
The dengue virus and the Zika virus share several characteristics. They both belong to the Flavivirus genus, they are both RNA viruses mainly transmitted by mosquitoes, and they have similar envelope proteins. Scientists from the Institut Pasteur, the CNRS and Imperial College London, who had previously identified antibodies capable of neutralizing the four types of dengue virus in an earlier study, decided to turn their attention to the Zika virus. "We wanted to see whether the antibodies isolated for dengue could be used to neutralize other flaviviruses, and Zika seemed like the best candidate," explained Félix Rey, Head of the Structural Virology Laboratory at the Institut Pasteur.
In this latest study, the scientists selected two antibodies capable of blocking the proliferation of the dengue virus. They began by isolating these antibodies in dengue patients, then presented them to the Zika virus. One of the antibodies proved to be highly efficient in neutralizing the Zika virus – even more so than for dengue – preventing it from infecting the cells it was cultured with. "We never expected to discover that the dengue virus and the Zika virus are so close that some antibodies produced against the dengue virus could also neutralize the Zika virus so potently" stressed Félix Rey.
The scientists then used crystallography to identify the antibody binding site on the Zika virus, and more specifically on the proteins in its viral envelope. Crystals containing the "antibody–envelope protein" complex were produced using the Institut Pasteur's Crystallography Platform. The scientists then used powerful X-rays produced by the synchrotron facilities in Saclay and Grenoble to create a 3D reconstruction of the precise location where the antibody binds to the envelope protein.
This revealed that the antibody binding site for the Zika virus is the same as for the dengue virus, raising the possibility of producing a vaccine that would stimulate the production of antibodies capable of binding to and neutralizing two types of virus at once. Although the Zika virus was not previously thought to be dangerous, neurological complications including Guillain-Barré syndrome have been observed in Zika patients in Brazil and French Polynesia. This virus is also the cause of severe fetal brain development defects (microcephaly), resulting in irreversible intellectual disability. "The antibodies could be used, for example, to protect pregnant women at risk of contracting the Zika virus, because there is currently no vaccine or treatment for this disease," concluded Félix Rey.
This work was funded by the institutions mentioned above and by the European Framework Programme (FP7) DENFREE, IBEID LabEx (Integrative Biology of Emerging Infectious Diseases) and the FlaviStem grant (ANR / FWF).
[1] Involved laboratories:
- Virology (CNRS/Institut Pasteur)
- Structural virology (CNRS/Institut Pasteur).
Source
Structural basis of potent Zika–dengue virus antibody cross neutralization, Nature, June 23, 2016
Giovanna Barba-Spaeth (1,2,#), Wanwisa Dejnirattisai (3,#), Alexander Rouvinski (1,2,#), Marie-Christine Vaney (1,2,#), Iris Medits (4), Arvind Sharma (1,2), Etienne Simon-Lorière (5,6), Anavaj Sakuntabhai (5,6), Van-Mai Cao-Lormeau (7), Ahmed Haouz (8), Patrick England (9), Karin Stiasny (4), Juthathip Mongkolsapaya (3,10), Franz X. Heinz (4*), Gavin R. Screaton (3*) and Félix A. Rey (1,2*)
(1) Institut Pasteur, Unité de Virologie Structurale, Département de Virologie, F-75724 Paris Cedex 15, France
(2) CNRS UMR 3569 Virologie, F-75724 Paris Cedex 15, France
(3) Division of Immunology and Inflammation, Department of Medicine, Hammersmith campus, Imperial College London, UK
(4) Department of Virology, Medical University of Vienna, Kinderspitalgasse 15, A-1095 Vienna, Austria
(5) Institut Pasteur, Unité de Génétique fonctionnelle des maladies infectieuses, Département de Génomes et Génétique, F-75724 Paris Cedex 15, France
(6) CNRS URA 3012, F-75724 Paris Cedex 15, France
(7) Unit of Emerging Infectious Diseases, Institut Louis Malardé, Papeete,Tahiti,French Polynesia
(8) Institut Pasteur, Plateforme de Cristallographie, Département de Biologie Structurale et Chimie, F-75724 Paris Cedex 15, France
(9) Institut Pasteur, Plateforme de Biophysique des Macromolécules et de leurs Interactions, Département de Biologie Structurale et Chimie, F-75724 Paris Cedex 15, France
(10) Dengue Hemorrhagic Fever Research Unit, Office for Research and Development, Siriraj Hospital, Faculty of Medicine, Mahidol University, Bangkok, Thailand
# These authors contributed equally and are listed in alphabetical order.





