Alzheimer’s disease: towards new diagnostic and therapeutic tracks

Nearly a million French people have Alzheimer’s disease. Many hopes have been raised by medical research in the field of neurodegenerative diseases, which are at the heart of the public’s concerns. But the brain still remains mysterious, with complex functioning, still largely unknown. At Institut Pasteur, brain connectivity and neurodegenerative diseases is one of the scientific priorities areas. Basic research in neuroscience is essential to find one day effective treatment options for brain diseases.
Forgetting your umbrella in a shop or losing your keys – these things can happen to us all, at any age. Memory lapses can often be explained by stress, tiredness or simple carelessness. With age, however, such incidents can become more frequent, and we can begin wondering if we may have the beginnings of Alzheimer's disease. But it is generally only when we notice a real impact on our day-to-day lives and our social interactions that we make an appointment to see our family physician. Alzheimer's is a multifactorial, progressive disorder. And although some 50 million people worldwide suffer from dementia, including 900,000 French people with Alzheimer's disease, diagnosing it remains a complex task.

What is Alzheimer's disease?
Alzheimer's disease is a progressive neurodegenerative disorder (see our fact sheet). It mainly affects memory but also has an impact on other cognitive functions linked to knowledge and involving language, reasoning, learning, etc. It generally leads to a loss of independence for sufferers. Alzheimer's disease is caused by a progressive degeneration of neurons, starting in the hippocampus (a brain structure that is essential for short-term memory). This degeneration spreads to other brain regions, then to the entire brain.
Alzheimer's disease involves the accumulation of two types of protein in the brain:
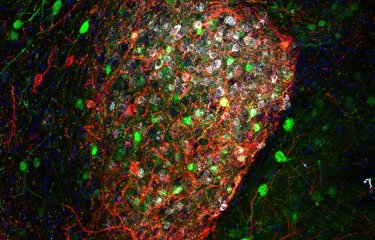
- Amyloid-beta peptide, which occurs naturally in humans, accumulates in an abnormal form in Alzheimer's patients, creating amyloid plaques (also known as senile plaques) that are toxic for neurons.
- Tau proteins, which play a role in neuronal architecture and stability, also build up abnormally, forming tangles that prevent neurons from communicating with each other.
The current theory is that the successive build-up of amyloid plaques and tau proteins results in the emergence of Alzheimer's symptoms.
But the mechanisms underpinning the development of Alzheimer's disease remain largely unknown. The purpose of basic research is to examine the underlying processes responsible for diseases, with a view to developing effective treatments.
At the Institut Pasteur, the Membrane Traffic and Pathogenesis Unit led by Chiara Zurzolo is investigating the mechanisms involved in the cell-to-cell transmission of amyloid proteins. The scientists have discovered direct connections between neurons that make this transmission possible. "The aim of our research is to develop tools that block the transmission of amyloid proteins, thereby delaying or preventing the progression of the disease," explains Chiara Zurzolo.

The Institut Pasteur pledges to improve understanding of brain connectivity for the particular benefit of patients with neurodegenerative diseases
Alzheimer’s, Parkinson’s… neurodegenerative diseases are at the forefront of public concern at a time when increasing numbers of patients and families are faced with these new health challenges. For almost 20 years, considerable hopes have been raised by scientific research in this vast field, but these initial hopes have often been dashed, for the brain remains an organ of mystery with complex workings that are still largely unknown.
Basic research is therefore vital if we are to have any hope of finding new potential avenues for effective treatment one day. Since the 1960s, the Institut Pasteur has been home to many fine neuroscience research teams whose discoveries have attracted international recognition – from the seminal work of Professor Jean-Pierre Changeux to the research of the current director of the Institut Pasteur’s Department of Neuroscience, Dr. David Digregorio. The Institut Pasteur has broad scientific expertise ranging from the investigation of genes to the observation of individual behavior (in animals and humans), research on proteins, synapses, neurons, neuronal networks, and brain regions, in both animal and human models. The Institut Pasteur recognized the importance of this research field in its 2019-2023 Strategic Plan, and is thus leveraging its wide-ranging expertise to improve our understanding of the complexity of brain function.
"Pasteurian" research projects focus on sensory deficits (deafness), neurodevelopmental disorders (autism) and psychiatric disorders (mood disorders and addiction), neurodegenerative diseases (Alzheimer’s and Parkinson’s diseases) and other neurological conditions (sepsis and neurovascular disorders). With their expertise in cell biology and state-of-the-art imaging (live microscopy, electron microscopy, super-resolution imaging) scientists also investigate the interneuronal transmission of misfolded proteins and the molecular mechanisms underpinning neurodegeneration. These conditions associated with brain connectivity are the result of damage to the brain’s neural network and to links between the brain and other organs. At the Institut Pasteur, thanks to the wide-ranging expertise developed from collaboration between different departments and disciplines (cell and developmental biology, immunology, computational biology, technological platform), it is possible to gain a comprehensive overview of these diseases, and thus integrate the central nervous system with the peripheral nervous system, immune system and microbial environment.
The importance of early diagnosis

We are now hoping to be able to develop the same technique for identifying the early stages of Alzheimer's disease in humans, so that we can visualize plaques and neurofibrillary tangles using MRI.
Pierre LafayeHead of the Antibody Engineering Platform at the Institut Pasteur
Since Alzheimer's disease is a progressive disorder, early diagnosis is vital. The Antibody Engineering Platform, led by Pierre Lafaye at the Institut Pasteur, has developed two new antibody types that are able to detect both extracellular targets (amyloid plaques) and intracellular targets characteristic of Alzheimer's disease.
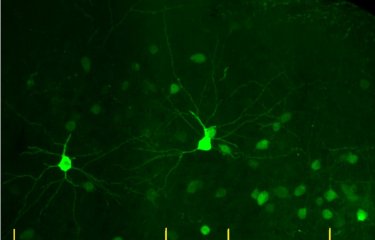
Using fluorescence imaging, the scientists have developed a technique that could be used to identify individuals developing Alzheimer's disease but not yet exhibiting any clinically visible symptoms. In animal models, the antibodies developed by the scientists bind with either amyloid-beta peptide or fibrillary tangles. On their own, amyloid-beta peptide and fibrillary tangles are not visible, but when they bind with the antibodies they can be located using fluorescence imaging. "We are now hoping to be able to develop the same technique for humans so that we can visualize plaques and neurofibrillary tangles using MRI," explains Pierre Lafaye.
The role of human genetics
Human genetics research has also led to major progress in our understanding of Alzheimer's disease, opening up new possibilities in terms of diagnostics and patient monitoring. The Integrative Neurobiology of Cholinergic Systems Unit, led by Uwe Maskos at the Institut Pasteur, is increasingly using human genetics techniques in a bid to be able to diagnose Alzheimer's at an early stage.

The unit has identified genetic alterations that predispose individuals to the disease. The scientists are working on a gene that is specific to humans. Individuals carrying this gene who lose one or two of its alleles appear to be predisposed to develop Alzheimer's disease at a younger age and in a more severe form. The scientists are now hoping to develop new possibilities for early diagnosis by testing for these genetic alterations in young people with family members affected by Alzheimer's disease and offering increased clinical monitoring for those who test positive. These individuals could also be chosen to participate in clinical trials. Initial inconclusive clinical trials have already been carried out on a gene coding for a subunit of the nicotinic receptor, the target of nicotine in the brain.

Basic research is vitally important. It is an essential tool that gives scientists a better understanding of a disease before they begin to envisage solutions to treat it. We have known about one of the risk-factor genes in Alzheimer's disease for 25 years. But even if the gene's involvement is clear, we have still not elucidated the mechanisms that link it to Alzheimer's disease.
Uwe MaskosHead of the Integrative Neurobiology of Cholinergic Systems Unit at the Institut Pasteur
This is of particular interest for the scientists since the subunit is completely blocked by a build-up of amyloid-beta peptide.
It is thought that the subjects in the clinical trials were already at too advanced a stage in the disease – hence the need to identify and diagnose patients at a younger age.
"Basic research is vitally important. We have known about one of the risk-factor genes in Alzheimer's disease for 25 years. But even if the gene's involvement is clear, we have still not elucidated the mechanisms that link it to Alzheimer's disease." The gene codes for a protein known as "lipoprotein", and one of the gene's variants, E4, gives rise to a 25% probability of developing Alzheimer's disease.
Another mutation has been described in 1 to 2% of the population in cases with a family history of the disease, where it can affect people as young as 35.
The acetylcholine nicotinic receptor, a target for future treatment
The unit led by Uwe Maskos is also studying the role of nicotinic receptors in Alzheimer's disease. These receptors are an initial target of amyloid-beta peptide and could form the basis of a new therapeutic approach. The idea is to intervene during the stage that occurs just after the initial phase of the disease, when treatments are still effective.
The first neurons that degenerate in Alzheimer's disease are those responsible for releasing acetylcholine. Based on these observations, several pharmaceutical laboratories have developed treatments that prevent the degradation of acetylcholine and increase the concentration in the brain. These treatments can stabilize the patient's condition for a number of years. "Although current treatments can increase the brain's concentration of acetylcholine, at a certain point it is no longer able to interact properly with its nicotinic receptor, since amyloid-beta peptide, which is partly responsible for Alzheimer's disease, has a high affinity with that receptor and interferes with the physiological interaction," explains Uwe Maskos. While insoluble deposits of the peptide form the infamous amyloid plaques, the soluble part of the peptide, which is invisible, interacts with the receptor. Since 2018, these treatments have no longer been eligible for refund under the French health system.
The impact of this peptide has been measured in an animal model using a genetic manipulation. By observing various behaviors and targeting the consequences of the amyloid-beta peptide in small brain structures such as the hippocampus, which is responsible for memory formation, the scientists were able to demonstrate the impact of Alzheimer's disease. Administering an antagonist with high affinity for the nicotinic receptor protected the animal models from the pathological effect of the peptide.
The scientists are now aiming to develop a treatment using an antagonist that is able to break the link between a subunit of the acetylcholine nicotinic receptor and the peptide.

The inflammatory role of microglial cells
By observing non-neuronal cells in the immune system and their role in Alzheimer's disease, the scientists identified another population of cells, microglial cells, that are involved in the condition. These cells are part of the brain's immune system and act like a "police force". They produce substances that promote the development of synapses, and they help detect the build-up of amyloid-beta peptide in the brain. They are derived from macrophages and are capable of "swallowing" excess amyloid-beta peptide. In Alzheimer's disease, scientists believe that glial cells end up being overwhelmed by the task. "We are trying to focus our efforts on an approach that would enable us to activate these microglial cells more strongly so that they are able to degrade amyloid-beta peptide and deal with it more effectively in brains that are beginning to be affected by Alzheimer's disease," explains Uwe Maskos.
But activating these cells also leads to the secretion of inflammatory cytokines, which are toxic for neighboring neurons. In carrying out their "police" work, microglial cells trigger neuroinflammation – which could also contribute to Alzheimer's disease. It is therefore vital to find a delicate balance between these therapeutic approaches.

The possibility of treatment based on nicotine
When it comes to Parkinson's and Alzheimer's disease, the scientific community now overwhelmingly accepts that nicotine has a positive effect.
In animal models, the administration of a strong dose of nicotine alone results in a reduction in amyloid plaques. Biochemical research demonstrates that nicotine is capable of "dissolving" these aggregates.
Clinical trials are currently being carried out to gage the tolerance of nicotine treatment, using nicotine patches, in individuals who have never smoked, for example.
The two primary objectives of scientists working on Alzheimer's disease are early diagnosis and more precise treatments, in the hope that one day, those with this condition can be treated sooner and more effectively.